Metyrosine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
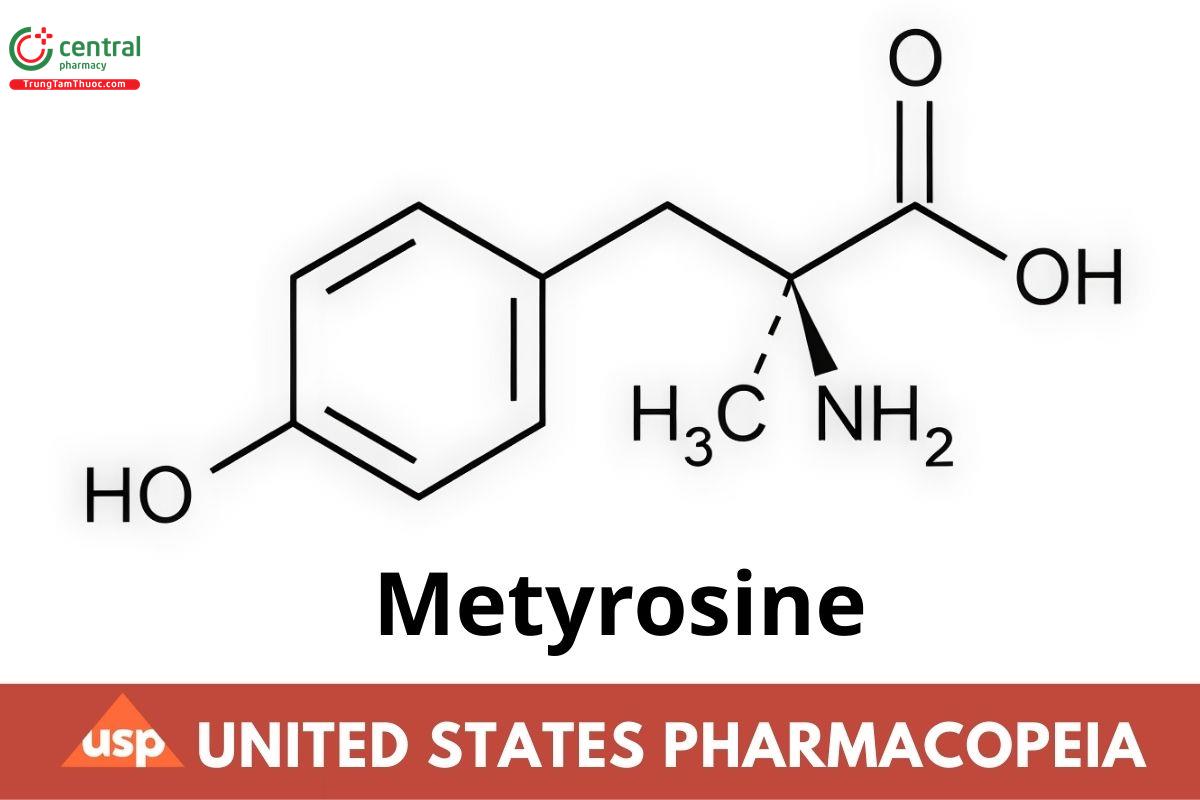
C10H13NO3 195.22
L-Tyrosine, a-methyl-, (-)-.
(-)-α-Methyl-L-tyrosine CAS RN: 672-87-7; UNII: DOQ0J0TPF7.
>> Metyrosine contains not less than 98.6 percent and not more than 101.0 percent of C10H13NO3, calculated on the dried basis.
1 Packaging and storage-
Preserve in well-closed containers.
2 USP REFERENCE STANDARDS (11)-
USP Metyrosine RS
Change to read:
3 Identification-
A: ▲Spectroscopic Identification Tests (197), Infrared Spectroscopy: 197M ▲(CN 1-May-2020).
B: ▲Spectroscopic Identification Tests (197), Ultraviolet-Visible Spectroscopy: 197U ▲(CN 1-May-2020).
Solution: 15 µg per mL.
Medium: 0.1 N hydrochloric acid.
Absorptivities at 224 nm, calculated on the dried basis, do not differ by more than 3.0%.
SPECIFIC ROTATION (781S): between +185° and +195" ( t = 30°, λ = 546nm, l = 0.5 dm).
Test solution: 5 mg per mL, in Diluent, with the aid of sonication if necessary. Prepare the Diluent as follows.
Solution A-Dissolve 20.0 g of anhydrous sodium acetate in about 150 mL of water in a 250-mL volumetric flask. Add 50.0 mL of glacial acetic acid, dilute with water to volume, and mix.
Solution B-Dissolve 62.5 g of cupric sulfate in water in a 200-mL volumetric flask, dilute with water to volume, and mix.
Diluent-Mix Solution A and Solution B in a 1000-mL volumetric flask, dilute with water to volume, and mix.
LOSS ON DRYING (731)-Dry it at a pressure not exceeding 5 mm of mercury at 100° for two hours: it loses not more than 1.0% of its weight.
RESIDUE ON IGNITION (281): not more than 0.1%.
4 Chromatographic purity-
Standard solutions-Dissolve USP Metyrosine RS in a solvent mixture of methanol and ammonium hydroxide (7:3) to obtain a solution having a concentration of 10 mg per mL (Standard solution A). Pipet 1 mL of Standard solution A into a 100-mL volumetric flask, dilute with the same solvent mixture to volume, and mix (Standard solution B). Pipet 5 mL of Standard solution B into a 10-mL volumetric flask, dilute with the same solvent mixture to volume, and mix (Standard solution C). Pipet 5 mL of Standard solution C into a 10-mL volumetric flask, dilute with the same solvent mixture to volume, and mix (Standard solution D).
Test solution-Dissolve Metyrosine in the solvent mixture of methanol and ammonium hydroxide (7:3) to obtain a solution having a concentration of 10 mg per mL.
Procedure-Apply 10-µL portions of Standard solutions A, B, C, and D and the Test solution to a suitable thin-layer chromatographic plate (see Chromatography (621)) coated with a 0.25-mm layer of chromatographic silica gel mixture and previously washed with methanol. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of n-propyl alcohol and ammonium hydroxide (7:3) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry the plate. Expose the plate to iodine vapors, and examine under short-wavelength UV light: the chromatogram shows principal spots at about the same RF value. Estimate the levels of any additional spots observed in the chromatogram of the Test solution by comparison with the spots in the chromatograms of Standard solutions B, C, and D: the sum of the intensities of any spots observed is not greater than that of the principal spot obtained from Standard solution B, corresponding to not more than 1%.
5 Assay-
Dissolve about 300 mg of Metyrosine, accurately weighed, in about 100 mL of glacial acetic acid, sonicate for about 5 minutes, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically, using a platinum ring electrode and a sleeve-type calomel electrode containing 0.1 N lithium perchlorate in glacial acetic acid (see Titrimetry (541)). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 19.52 mg of C10H13NO3.


