Metyrapone
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
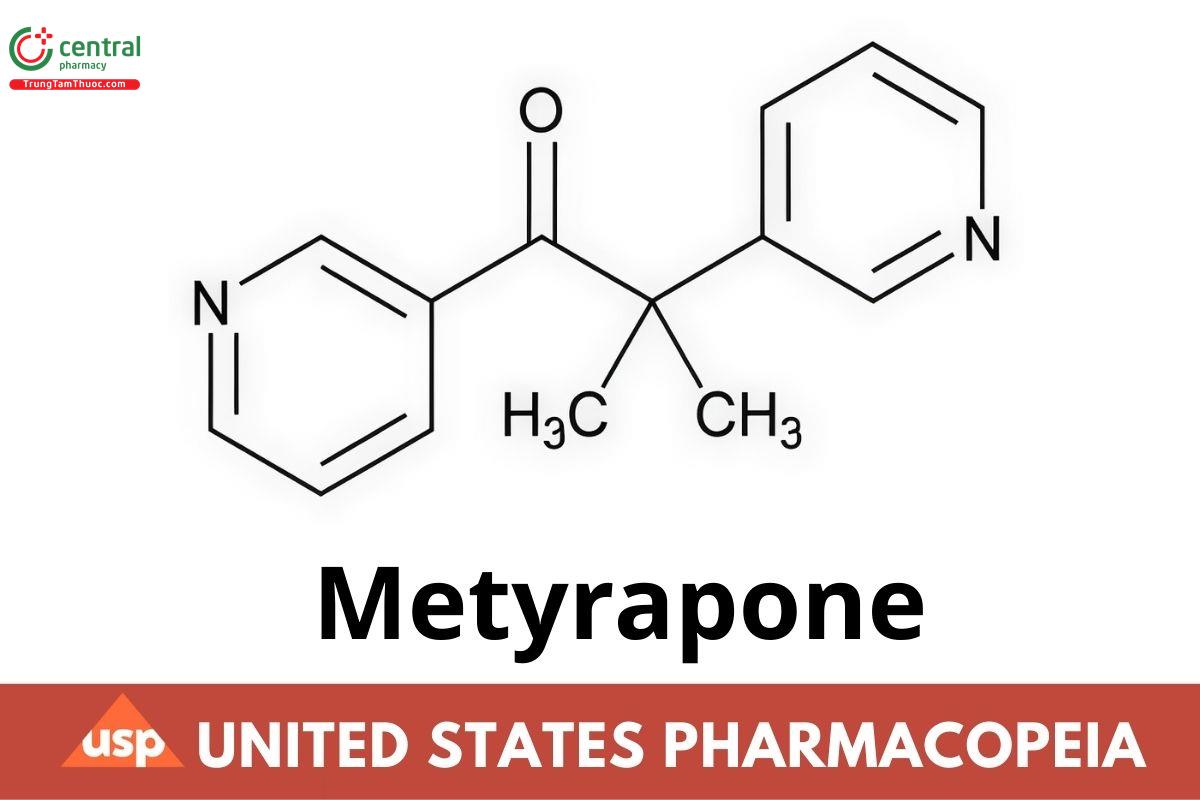
C14H14N2O 226.27
1-Propanone, 2-methyl-1,2-di-3-pyridinyl-;
2-Methyl-1,2-di-3-pyridyl-1-propanone CAS RN®: 54-36-4; UNII: ZS9KD92H6V.
1 DEFINITION
Metyrapone contains NLT 98.0% and NMT 102.0% of metyrapone (C14H14N2O), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M ▲(CN 1-MAY-2020)
Change to read:
B. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 1970 ▲(CN 1-MAY-2020)
Sample solution: 10 µg/mL in 1 N sulfuric acid
Acceptance criteria: Meets the requirements
3 ASSAY
PROCEDURE
Diluent: 1 N sulfuric acid
Standard solution: 10 µg/mL of USP Metyrapone RS in Diluent
Sample solution: 10 µg/mL of Metyrapone in Diluent
Instrumental conditions
Mode: UV
Analytical wavelength: 260 nm
Cell: 1 cm
Blank: Diluent
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of metyrapone (C14H14N2O) in the portion of Metyrapone taken:
Result = (AU/AS) × (CS/CU) × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of USP Metyrapone RS in the Standard solution (µg/mL)
CU = concentration of Metyrapone in the Sample solution (µg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%
4.2 ORGANIC IMPURITIES
Standard stock solution: 0.2 mg/mL of USP Metyrapone RS in methanol
Standard solution A: 40 µg/mL of USP Metyrapone RS in methanol
Standard solution B: 20 µg/mL of USP Metyrapone RS in methanol
Sample solution: 20 mg/mL of Metyrapone in methanol
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 5 µL
Developing solvent system: Chloroform and methanol (48:3)
Analysis
Samples: Standard stock solution, Standard solution A, Standard solution B, and Sample solution
Apply each of the Samples separately to the TLC plate. Position the plate in a chromatographic chamber, and develop the chromatograms in the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry under a stream of nitrogen for about 10 min. Position the dried plate once again in the same chromatographic chamber, and again develop the chromatograms until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and dry under a current of warm air for about 15 min. Examine the plate under short-wavelength UV light, and compare the intensities of any secondary spots observed in the chromatogram of the Sample solution with those of the principal spots in the chromatograms of the Standard solutions.
Acceptance criteria: The intensity of any secondary spot from the Sample solution is NMT the principal spot from Standard solution A (0.2%), and the sum of the intensities of the secondary spots from the Sample solution corresponds to NMT 1.0%.
5 SPECIFIC TESTS
LOSS ON DRYING (731)
Analysis: Dry a sample under vacuum at room temperature for 6 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, protected from heat and light.
USP REFERENCE STANDARDS (11)
USP Metyrapone RS


