Metoprolol Succinate Extended-Release Tablets
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
- DEFINITION
- IDENTIFICATION
- ASSAY
- PERFORMANCE TESTS
- Test 1
- Test 2: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 2.
- Test 4: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 4.
- Test 5: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 5.
- Test 7: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 7.
- Test 8: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 8.
- Test 9: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 9.
- Test 10: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 10.
- Test 11: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 11.
- Test 12: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 12.
- Test 13: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 13.
- IMPURITIES
- ADDITIONAL REQUIREMENTS
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
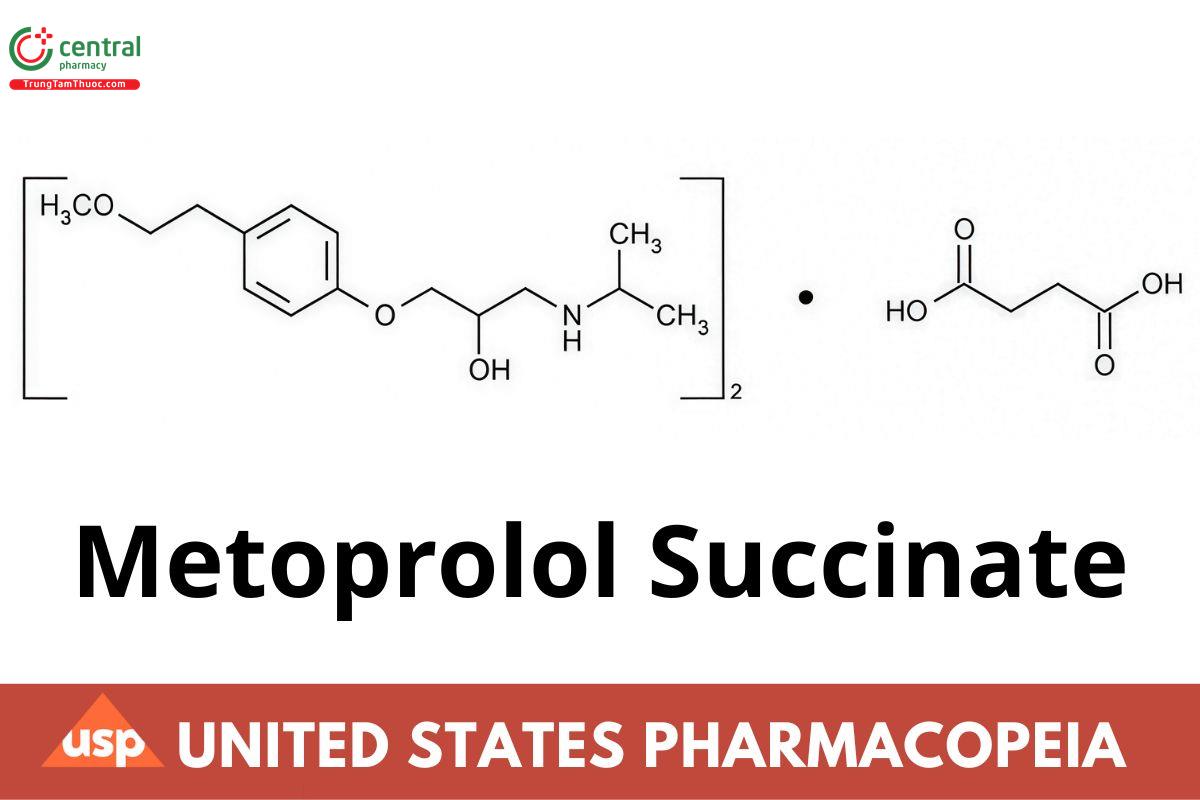
Metoprolol Succinate Extended-Release Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of metoprolol succinate
[(C15H25NO3)2 · C4H6O4]
2 IDENTIFICATION
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. The UV spectrum of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Buffer: Mix 50 mL of 1 M monobasic sodium phosphate and 8.0 mL of 1 M phosphoric acid, and dilute with water to 1000 mL. If necessary, adjust with 1 M monobasic potassium phosphate or 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: 0.05 mg/mL of USP Metoprolol Succinate RS in Mobile phase
Sample stock solution: Nominally 1 mg/mL of metoprolol succinate prepared as follows. Transfer a suitable number of Tablets to a suitable volumetric flask, add about 5 mL of water, and allow the Tablets to disintegrate. Add a volume of alcohol to fill 30% of the flask volume, and shake for 30 min. Add a portion of 0.1 N hydrochloric acid to fill 50% of the flask volume, and shake for an additional 30 min. Dilute with 0.1 N hydrochloric acid to volume. Filter, and discard the first 10 mL of the filtrate.
Sample solution: Nominally 0.05 mg/mL of metoprolol succinate from the Sample stock solution in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm. For Identification B, use a diode array detector in the range of 200-400 nm.
Column: 4-mm x 12.5-cm; 5-µm packing LZ
Flow rate: 1 mL/min
Injection volume: 40 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of metoprolol from the Sample solution
rS= peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
CU = nominal concentration of metoprolol succinate in the Sample solution (mg/mL)
Acceptance criteria: 90.0%-110.0%
4 PERFORMANCE TESTS
Change to read:
DISSOLUTION (711).
4.1 Test 1
Medium: pH 6.8 phosphate buffer (see Reagents, Indicators, and Solutions-Buffer Solutions); 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, and 20 h
Buffer and Mobile phase: Prepare as directed in the Assay.
Standard solution: A known concentration of USP Metoprolol Succinate RS in Medium
Sample solution: Pass a portion of the solution under test through a suitable filter.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4-mm x 12.5-cm; 5-µm packing LZ
Flow rate: 1 mL/min
Injection volume: 40 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point.
Tolerances: See Table 1.
Table 1
| Time (h) | Amount Dissolved (%) |
| 1 | NMT 25 |
| 4 | 20-40 |
| 8 | 40-60 |
| 20 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.2 Test 2: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 2.
Medium: Simulated gastric fluid without enzyme, pH 1.2; 500 mL
Apparatus 2: 75 rpm
Times: 1, 4, 8, and 20 h
Buffer: 1 M monobasic sodium phosphate, 1 M phosphoric acid, and water (50:8:942). If necessary, adjust with 1 M monobasic sodium phosphate or 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (250:750)
Standard solution: Prepare a solution of USP Metoprolol Succinate RS in Medium as directed in Table 2.
Table 2
| Tablet Strength (mg) | Concentration (mg/mL) |
| 200 | 0.380 |
| 100 | 0.190 |
| 50 | 0.095 |
| 25 | 0.048 |
Sample solution: Pass the solution under test through a suitable filter.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm x 12.5-cm; 4-µm packing LZ
Flow rate: 1 mL/min.
Injection volume: See Table 3.
Table 3
| Tablet Strength (mg) | Volume (μL) |
| 25 | 40 |
| 50 | 20 |
| 100 | 10 |
| 200 | 5 |
Run time: NLT 2 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 1500 theoretical plates
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved in Medium at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved (Q), at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = {[C2 x (V - VS)] + (C1 x VS)) x (1/L) x 100
Result3 = ((C3 x [V - (2 x VS)] + [(C2 + C1) x VS) x (1/L) x 100
Result4 = ({C4 x [V - (3 x VS)] + [(C3 + C2 + C1) x VS) x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point (i) (mg/mL)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn from the Medium (mL)
Tolerances: See Table 4.
Table 4
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | NMT 20 |
| 2 | 4 | 20–40 |
| 3 | 8 | 55–85 |
| 4 | 20 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.3 Test 4: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 4.
Medium: Phosphate buffer, pH 6.8 (dissolve 6.8 g of monobasic potassium phosphate and 0.93 g of sodium hydroxide in 1 L of water: adjust with a sodium hydroxide solution to a pH of 6.8); 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, and 24 h
Buffer: 5.0 mL/L of triethylamine in water. Adjust with phosphoric acid to a pH of 3.0.
Mobile phase: Methanol and Buffer (40:60)
Standard solution: Prepare a solution of USP Metoprolol Succinate RS in Medium as directed in Table 5.
Table 5
| Tablet Strength (mg) | Concentration (mg/mL) |
| 200 | 0.4 |
| 100 | 0.2 |
| 50 | 0.1 |
| 25 | 0.05 |
Sample solution: Withdraw a 10-mL aliquot at each time point. Pass the solution under test through a suitable filter of 0.45-µm pore size. Replace the portion withdrawn with an equal volume of Medium.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 223 nm
Column: 4.6-mm x 25-cm; 5-µm packing 11
Column temperature: 30°
Flow rate: 1.5 mL/min
Injection volume: 5 µL
Run time: NLT 2 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved in Medium at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution s
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved (Q), at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = {(C2 x V)+ (C1 x VS)} x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS)} x (1/L) x 100
Result4 = {(C4 x V) + [(C3 + C2 + C1) x VS)} x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point (i) (mg/mL)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn from the Medium (mL) S
Tolerances: See Table 6.
Table 6
| Time Point (1) | Time (h) | Amount Dissolved (Tablets labeled 25 mg) (%) | Amount Dissolved (Tablets labeled 50, 100, and 200 mg) (%) |
| 1 | 1 | NMT 20 | NMT 20 |
| 2 | 4 | 20-40 | 15-35 |
| 3 | 8 | 42-67 | 38-64 |
| 4 | 24 | NLT 80 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.4 Test 5: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 5.
Medium: Phosphate buffer, pH 6.8 (dissolve 27.22 g of monobasic potassium phosphate and 3.6 g of sodium hydroxide in 4 L of water, adjust with 1 N sodium hydroxide or phosphoric acid to a pH of 6.8); 500 mL
Apparatus 2: 50 rpm, with sinkers
Times: 1, 4, 8, and 20 h
Buffer: Transfer 3.0 mL of triethylamine and 1.0 mL of phosphoric acid to a 1000-mL volumetric flask that contains 600 mL of water. Dilute with water to volume.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: Prepare a solution of USP Metoprolol Succinate RS in Medium as directed in Table 7.
Table 7
| Tablet Strength (mg) | Concentration (mg/mL) |
| 200 | 0.2 |
| 100 | 0.2 |
| 50 | 0.05 |
| 25 | 0.05 |
Sample solution: Withdraw a 10-mL aliquot at each time point. Pass the solution under test through a suitable filter of 0.45-µm pore size. Replace the portion withdrawn with an equal volume of Medium.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 15-cm; 5-µm packing L7
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 40 µL for 25 and 50 mg; 10 µL for 100 and 200 mg
Run time: NLT 2 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 3.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved in Medium at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution s
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved (Q), at each time point (1):
Result1 = C1 x V x (1/L) x 100
Result2 = {(C2 x V)+ (C1 x VS)} x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS)} x (1/L) x 100
Result4 = {(C4 x V) + [(C3 + C2 + C1) x VS)} x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point (i) (mg/mL)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn from the Medium (mL)
Tolerances: See Table 8.
Table 8
| Time Point | Time (h) | Amount Dissolved (%) |
| 1 | 1 | NMT 10 |
| 2 | 4 | 5-30 |
| 3 | 8 | 30-55 |
| 4 | 20 | NLT 75 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.5 Test 7: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 7.
Medium: Phosphate buffer, pH 6.8 (dissolve 6.8 g of monobasic potassium phosphate and 0.9 g of sodium hydroxide in 1 L of water, adjust with 1 N sodium hydroxide or 1 M phosphoric acid to a pH of 6.8), deaerated; 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, 12, and 24 h for Tablets labeled 25 and 50 mg and 1, 4, 8, and 20 h for Tablets labeled 100 and 200 mg
Buffer: Mix 50 mL of 1 M monobasic sodium phosphate dihydrate and 8.0 mL of 1 M phosphoric acid, and dilute with water to 1000 mL.
Adjust with 1 M monobasic sodium phosphate dihydrate or 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (22:78)
Standard solution: Known concentrations of USP Metoprolol Succinate RS in Medium are listed in Table 9.
Table 9
| Tablet Strength (mg) | Concentration (mg/mL) |
| 100 | 0.2 |
| 50/200 | 0.1 |
| 25 | 0.05 |
Sample solution: Withdraw an 8-mL aliquot of the solution under test at each time point. For Tablets labeled 25, 50, and 100 mg, no further dilution is required. For Tablets labeled 200 mg, transfer 5.0 mL of the aliquot withdrawn to a 20-ml volumetric flask, and dilute with Medium to volume. Centrifuge, if needed. Pass the supernatant through a suitable filter of 0.45-µm pore size.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 15-cm; 5-µm packing 17
Column temperature: 30°
Flow rate: 0.9 mL/min
Injection volume: 10 µL
Run time: NLT 2.6 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved at each time point (1):
Result = (rU/rS) × CS x D
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
D = dilution factor for the Sample solution
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved (Q), at each time point (i): 15
Result1 = C1 x V x (1/L) x 100
Result2 = {[C2 x (V - VS)] + (C1 x VS)) x (1/L) x 100
Result3 = ((C3 x [V - (2 x VS)] + [(C2 + C1) x VS) x (1/L) x 100
Result4 = ({C4 x [V - (3 x VS)] + [(C3 + C2 + C1) x VS) x (1/L) x 100
Result5 = ({C5 x [V - (4 x VS)] + [(C4 + C3 + C2 + C1) x VS) x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point (i) (mg/mL)
V = volume of Medium, 500 mL
L = label claim of metoprolol succinate (mg/Tablet)
VS = volume of the sample withdrawn at each time point, 8 mL
Tolerances: See Table 10 and Table 11.
Table 10
| Time Point (i) | Time (h) | Amount Dissolved (Tablets labeled 25 and 50 mg) (%) |
| 1 | 1 | NMT 15 |
| 2 | 4 | 10-30 |
| 3 | 8 | 35-55 |
| 4 | 12 | 55-75 |
| 5 | 24 | NLT 80 |
Table 11
| Time Point (i) | Time (h) | Amount Dissolved (Tablets labeled 25 and 50 mg) (%) |
| 1 | 1 | NMT 17 |
| 2 | 4 | 17–37 |
| 3 | 8 | 42–62 |
| 4 | 20 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.6 Test 8: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 8.
Medium: Phosphate buffer, pH 6.8; 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, and 24 h
Solution A: Dissolve 156.0 g of monobasic sodium phosphate dihydrate in 1 L of water.
Solution B: Dissolve 68.2 mL of phosphoric acid in 1 L of water.
Solution C: Dissolve 136.0 g of monobasic potassium phosphate in 1 L of water.
Buffer: Mix 50 mL of Solution A and 8.0 mL of Solution B, and dilute with water to 1000 mL. Adjust with Solution C or Solution B to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: 0.05 mg/mL of USP Metoprolol Succinate Succinate RS in Medium
Sample solution: At the Times specified, withdraw a known volume of the solution under test and replace with an equal volume of fresh Medium. Pass through a suitable filter of 0.45-µm pore size, discarding the first 1 mL of filtrate. Dilute the filtrate with Medium to a concentration similar to that of the Standard solution.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm x 12.5-cm; 5-µm packing LZ
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 40 µL
Run time: NLT 1.8 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate [(C15H25NO3)2 · C4H6O4] in t point (i):
Result = (rU/rS) × CS x D
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
D = dilution factor for the Sample solution
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = {(C2 x V)+ (C1 x VS)} x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS)} x (1/L) x 100
Result4 = {(C4 x V) + [(C3 + C2 + C1) x VS)} x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point i (mg/mL)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (1) and replaced with Medium (mL)
Tolerances: See Table 12.
Table 12
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | NMT 20 |
| 2 | 4 | 20–40 |
| 3 | 8 | 42–62 |
| 4 | 24 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.7 Test 9: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 9.
Medium: Phosphate buffer, pH 6.8; 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, 20, and 24 h
Buffer: Mix 50 mL of 1 M monobasic sodium phosphate and 8.0 mL of 1 M phosphoric acid, and dilute with water to 1000 mL. Adjust with 1 M monobasic sodium phosphate or 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: 0.05 mg/mL of USP Metoprolol Succinate RS in Medium
Sample solution: At the times specified, withdraw a portion of the solution under test, and pass the solution under test through a suitable filter of 0.45-µm pore size.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4-mm x 12.5-cm; 5-µm packing LZ
Flow rate: 1 mL/min
Injection volume: 10 µL
Run time: NLT 2 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved at each time point (1):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution u
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL) CS
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = {[C2 x (V - VS)] + (C1 x VS)) x (1/L) x 100
Result3 = ((C3 x [V - (2 x VS)] + [(C2 + C1) x VS) x (1/L) x 100
Result4 = ({C4 x [V - (3 x VS)] + [(C3 + C2 + C1) x VS) x (1/L) x 100
Result5 = ({C5 x [V - (4 x VS)] + [(C4 + C3 + C2 + C1) x VS) x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point (i) (mg/mL)
V = volume of Medium, 500 mL
L = label claim of metoprolol succinate (mg/Tablet)
VS = volume of the sample withdrawn at each time point (i) (mL)
Tolerances: See Table 13.
Table 13
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | NMT 20 |
| 2 | 4 | 20–40 |
| 3 | 8 | 40–60 |
| 4 | 20 | 68–88 |
| 5 | 24 | NLT 75 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711). Acceptance Table 2.▲(RB 13-Sep-2024)
4.8 Test 10: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 10.
Medium: Phosphate buffer. pH 6.8; 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, and 20 h
Buffer: Mix 50 mL of 1 M monobasic sodium phosphate and 8.0 mL of 1 M phosphoric acid, and dilute with water to 1000 mL. If necessary, adjust with 1 M monobasic potassium phosphate or 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: (L/500) mg/mL of USP Metoprolol Succinate RS in Medium, where L is the label claim of metoprolol succinate in mg/Tablet. Sonicate to dissolve, if necessary.
Sample solution: At the Times specified, withdraw an aliquot and replace the portion withdrawn with an equal volume of Medium. Pass the solution under test through a suitable filter of 0.10-µm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm x 12.5-cm; 5-µm packing LZ
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: See ▲Table 14.
Table 14 ▲(RB 13-Sep-2024)
| Tablet Strength (mg) | Volume (μL) |
| 25 | 40 |
| 50 | 20 |
| 100/200 | 10 |
Run time: NLT 2.1 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved in Medium at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = {(C2 x V)+ (C1 x VS)} x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS)} x (1/L) x 100
Result4 = {(C4 x V) + [(C3 + C2 + C1) x VS)} x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time point i (mg/mL)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (1) and replaced with Medium (mL)
Tolerances: See ▲Table 15.
Table 15 ▲(RB 13-Sep-2024)
| Time Point (1) | Time (h) | Amount Dissolved (Tablets labeled 25 and 50 mg) (%) | Amount Dissolved (Tablets labeled 100 and 200 mg) (%) |
| 1 | 1 | NMT 15 | NMT 15 |
| 2 | 4 | 12–32 | 12–32 |
| 3 | 8 | 36–56 | 33–53 |
| 4 | 20 | NLT 80 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.9 Test 11: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 11.
Medium: Phosphate buffer, pH 6.8 (Dissolve 6.8 g of monobasic potassium phosphate and 0.9 g of sodium hydroxide in 1 L of water; adjust with a sodium hydroxide solution or phosphoric acid to a pH of 6.8.); 500 mL
Apparatus 2: 50 rpm
Times: 2, 8, 16, and 30 h
Buffer: Dissolve 1.7 g of monobasic sodium phosphate in 1000 mL of water. Adjust with phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: 0.2 mg/mL of USP Metoprolol Succinate RS in Medium. Sonicate to dissolve, if necessary.
Sample solution: At the Times specified, withdraw a portion of the solution under test and pass through a suitable filter of 45-µm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 7.5-cm; 5-µm packing LZ
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 1.5 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point (i):
Result1 = C1 x V x (1/L) x 100
Result2 = {[C2 x (V - VS)] + (C1 x VS)) x (1/L) x 100
Result3 = ((C3 x [V - (2 x VS)] + [(C2 + C1) x VS) x (1/L) x 100
Result4 = ({C4 x [V - (3 x VS)] + [(C3 + C2 + C1) x VS) x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time pointi (mg/mL)
V = volume of Medium, 500 mL
L = label claim of metoprolol succinate (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (i) from the Medium (mL)
Tolerances: See ▲Table 16.
Table 16 ▲(RB 13-Sep-2024)
| Time Point (1) | Time (h) | Amount Dissolved (Tablets labeled 25 and 50 mg) (%) | Amount Dissolved (Tablets labeled 100 and 200 mg) (%) |
| 1 | 2 | NMT 10 | NMT 10 |
| 2 | 8 | 20–40 | 10–30 |
| 3 | 16 | 65–85 | 50–70 |
| 4 | 30 | NLT 85 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.10 Test 12: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 12.
Medium: Phosphate buffer, pH 6.8 (Dissolve 6.8 g of monobasic potassium phosphate and 0.9 g of sodium hydroxide in 1 L of water; adjust with 2 N sodium hydroxide solution to a pH of 6.8.); 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, and 20 h
Buffer: Dissolve 7.8 g monobasic sodium phosphate dihydrate in 1000 mL of water. Adjust with phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: (L/500) mg/mL of USP Metoprolol Succinate RS in Medium, where L is the label claim of metoprolol succinate in mg/Tablet. Sonicate to dissolve, if necessary.
Sample solution: At the Times specified, withdraw an aliquot and replace the portion withdrawn with an equal volume of Medium. Pass through a suitable filter of 0.45-µm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 15-cm; 5-µm packing 17
Flow rate: 1 mL/min
Injection volume: 40 µL for 25 and 50 mg; 10 µL for 100 and 200 mg
Run time: NLT 1.8 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved in Medium at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point (1):
Result1 = C1 x V x (1/L) x 100
Result2 = {(C2 x V)+ (C1 x VS)} x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS)} x (1/L) x 100
Result4 = {(C4 x V) + [(C3 + C2 + C1) x VS)} x (1/L) x 100
Ci= concentration of metoprolol succinate in the portion of sample withdrawn at time point i(mg / m * L)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (i) and replaced with Medium (mL)
Tolerances: See ▲Table 17.
Table 17▲(RB 13-Sep-2024)
| Time (h) | Amount Dissolved (%) |
| 1 | NMT 25 |
| 4 | 15–35 |
| 8 | 35–55 |
| 20 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
4.11 Test 13: If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 13.
Medium: Phosphate buffer, pH 6.8 (Dissolve 6.8 g of monobasic potassium phosphate in 250 mL of water, add 112 mL of 0.2 N sodium hydroxide and 500 mL of water, adjust with 0.2 N sodium hydroxide or 2 N hydrochloric acid to a pH of 6.8. Dilute with water to 1000 mL.); 500 mL
Apparatus 2: 50 rpm
Times: 1, 4, 8, and 20 h for Tablets labeled 25 and 50 mg; 1, 4, 10, and 24 h for Tablets labeled 100 and 200 mg
Buffer: 1 M monobasic sodium phosphate, 1 M phosphoric acid, and water (50:8:942). If necessary, adjust with 1 M monobasic sodium phosphate or 1 M phosphoric acid to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard solution: (L/500) mg/mL of USP Metoprolol Succinate RS in Medium, where L is the label claim of metoprolol succinate in mg/Tablet. Sonicate to dissolve, if necessary.
Sample solution: At the Times specified, withdraw an aliquot and replace the portion withdrawn with an equal volume of Medium. Pass through a suitable filter of 0.45-µm pore size, discarding an appropriate volume of filtrate so that a consistent result can be obtained.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 15-cm; 5-µm packing LZ
Flow rate: 1 mL/min
Injection volume: 10 µL
Run time: NLT 2.0 times the retention time of metoprolol
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C) of metoprolol succinate dissolved in Medium at each time point (i):
Result = (rU/rS) × CS
rU = peak response of metoprolol from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at each time point (i): 15 25
Result1 = C1 x V x (1/L) x 100
Result2 = {(C2 x V)+ (C1 x VS)} x (1/L) x 100
Result3 = {(C3 x V) + [(C2 + C1) x VS)} x (1/L) x 100
Result4 = {(C4 x V) + [(C3 + C2 + C1) x VS)} x (1/L) x 100
Ci = concentration of metoprolol succinate in the portion of sample withdrawn at time pointi (mg/mL)
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (i) and replaced with Medium (mL)
Tolerances: See ▲Table 18 and Table 19.
Table 18 ▲(RB 13-Sep-2024)
| Time Point (1) | Time (h) | Amount Dissolved (Tablets labeled 25 mg) (%) | Amount Dissolved (Tablets labeled 50 mg) (%) |
| 1 | 1 | NMT 20 | NMT 20 |
| 2 | 4 | 15–35 | 15–35 |
| 3 | 8 | 38–58 | 35–55 |
| 4 | 20 | NLT 80 | NLT 80 |
Table 19 ▲(RB 13-Sep-2024)
| Time Point (1) | Time (h) | Amount Dissolved (Tablets labeled 100 and 200 mg) (%) |
| 1 | 1 | NMT 20 |
| 2 | 4 | 10–30 |
| 3 | 10 | 40–60 |
| 4 | 24 | NLT 80 |
The percentages of the labeled amount of metoprolol succinate [(C15H25NO3)2 · C4H6O4] dissolved at the times specified conform to Dissolution (711), Acceptance Table 2.
UNIFORMITY OF DOSAGE UNITS (905): Meet the requirements
5 IMPURITIES
Change to read:
ORGANIC IMPURITIES
Buffer: 1.15 mL of phosphoric acid in 2 L of water. Add 2.6 g of sodium dodecyl sulfate. Sonicate to dissolve.
Solution A: Methanol and Buffer (30:70)
Solution B: Acetonitrile and Buffer (75:25)
Mobile phase: See ▲Table 20.
Table 20 ▲(RB 13-Sep-2024)
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 65 | 35 |
| 20 | 65 | 35 |
| 25 | 40 | 60 |
| 30 | 35 | 65 |
| 35 | 35 | 65 |
| 37 | 65 | 35 |
| 50 | 65 | 35 |
Diluent: Acetonitrile and Buffer (40:60)
System suitability solution: 3 µg/mL of USP Metoprolol Related Compound A RS and 1 mg/mL of USP Metoprolol Succinate RS in Diluent
Standard solution: 3 µg/mL of USP Metoprolol Succinate RS in Diluent
Sensitivity solution: 0.5 µg/mL of USP Metoprolol Succinate RS from Standard solution in Diluent
Sample solution: Nominally 1 mg/mL of metoprolol succinate from Tablets prepared as follows. Transfer a portion of finely powdered Tablets (NLT 20), equivalent to 50 mg of metoprolol succinate, to a 50-ml volumetric flask. Add Diluent to fill 60% of the flask volume and sonicate for 30 min with intermittent shaking. Dilute with Diluent to volume. Pass the solution through a suitable filter of 0.45-µm pore size.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 223 nm
Column: 4.6-mm x 15-cm; 5-µm packing L1
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[ NOTE-The relative retention times for succinic acid (the counter ion), metoprolol related compound A, and metoprolol are about 0.1, 0.83, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between metoprolol related compound A and metoprolol, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of any unspecified impurity in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of any unspecified impurity from the Sample solution
rS = peak response of metoprolol from the Standard solution
CS = concentration of USP Metoprolol Succinate RS in the Standard solution (µg/mL)
CU = nominal concentration of metoprolol succinate in the Sample solution (µg/mL)
Acceptance criteria: The reporting threshold is 0.05%.
Any unspecified impurity: NMT 0.20%
Total impurities: NMT 0.75%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, and store at controlled room temperature.
LABELING: Label it to indicate the content of metoprolol succinate and its equivalent, expressed as metoprolol tartrate [(C15H25NO3)2 · C4H6O4]
When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
USP REFERENCE STANDARDS (11).
USP Metoprolol Related Compound A RS
1-Ethylamino-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol.
C14H23NO2 253.34
USP Metoprolol Succinate RS


