Methylparaben Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
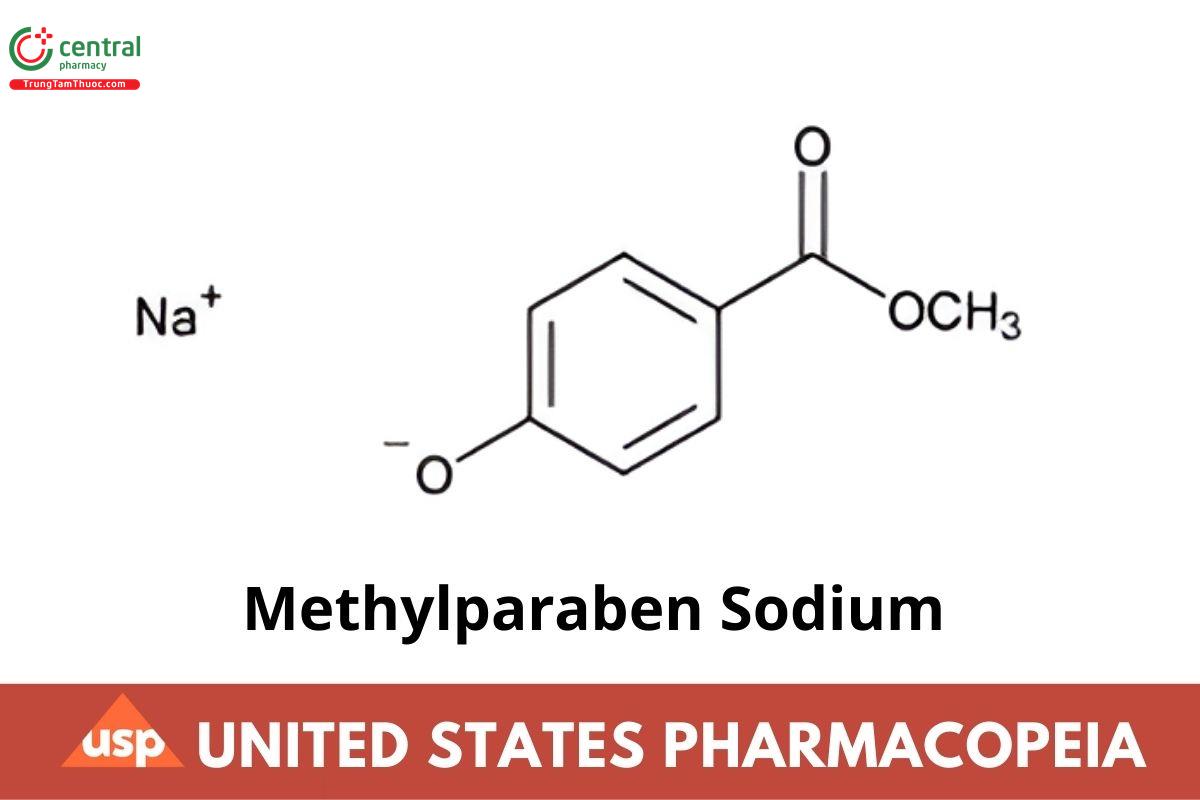
C8H7NaO3 174.13
Benzoic acid, 4-hydroxy-, methyl ester, sodium salt;
Methyl p-hydroxybenzoate, sodium salt;
Sodium 4-methoxycarbonylphenolate CAS RN®: 5026-62-0.
1 DEFINITION
Methylparaben Sodium contains NLT 95.0% and NMT 102.0% of methylparaben sodium (C8H7NaO3), calculated on the anhydrous basis.
2 IDENTIFICATION
A.
Standard: 0.5 g of USP Methylparaben RS
Sample: 0.5 g
Analysis: Dissolve the Sample in 5 mL of water. Acidify with hydrochloric acid, and filter the resulting precipitate. Wash the precipitate with water, and dry it over silica gel for 5 h. Repeat with the Standard.
Acceptance criteria: The IR absorption spectrum of a Mineral oil dispersion of the Sample exhibits maxima only at the same wavelengths as those of a similar preparation of the Standard.
B.
Sample solution: Ignite 0.3 g of Methylparaben Sodium, cool, and dissolve the residue in about 3 mL of 3 N hydrochloric acid.
Acceptance criteria: A platinum wire dipped in the Sample solution imparts an intense, persistent yellow color to a nonluminous flame.
3 ASSAY
Procedure
Mobile phase: Methanol and a 6.8 g/L solution of potassium dihydrogen phosphate (65:35, v/v)
System suitability solution: 5.0 μg/mL each of p-hydroxybenzoic acid and USP Methylparaben RS in Mobile phase
Standard solution: Dissolve 50.0 mg of USP Methylparaben RS in 2.5 mL of methanol, and dilute with Mobile phase to 50.0 mL. Dilute 10.0 mL of this solution with Mobile phase to 100.0 mL.
Sample solution: Dissolve 50.0 mg of Methylparaben Sodium in 2.5 mL of methanol, and dilute with Mobile phase to 50.0 mL. Dilute 10.0 mL of this solution with Mobile phase to 100.0 mL.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 272 nm
Column: 4.6-mm × 15-cm; 5-μm packing L1
Flow rate: 1.3 mL/min
Injection volume: 10 μL
Run time: About 5 times the retention time of the methylparaben peak
System suitability
Samples: System suitability solution and Standard solution
[Note - The retention time for methylparaben is about 2.2 min; the relative retention times for p-hydroxybenzoic acid and methylparaben are about 0.7 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the p-hydroxybenzoic acid and methylparaben peaks, System suitability solution
Relative standard deviation: NMT 0.85% for six injections, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of methylparaben sodium (C8H7NaO3) in the portion of Methylparaben Sodium taken:
Result = P × (rU/rS) × (CS/CU) × (Mr1/Mr2)
P = labeled purity of USP Methylparaben RS expressed as a percentage
rU = peak area of methylparaben from the Sample solution
rS = peak area of methylparaben from the Standard solution
CS = concentration of methylparaben in the Standard solution
CU = concentration of Methylparaben Sodium in the Sample solution
Mr1 = molecular weight of methylparaben sodium, 174.13
Mr2 = molecular weight of methylparaben, 152.15
Acceptance criteria: 95.0%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 Related Compounds
Mobile phase, System suitability solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: Dilute 1.0 mL of the Sample solution with Mobile phase to 20.0 mL. Dilute 1.0 mL of this solution with Mobile phase to 10.0mL.
System suitability
Sample: System suitability solution
[Note - The retention time for methylparaben is about 2.2 min; the relative retention times for p-hydroxybenzoic acid and methylparaben are about 0.7 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the p-hydroxybenzoic acid and methylparaben peaks
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria
p-Hydroxybenzoic acid: NMT 3.0%; the peak area in the Sample solution, multiplied by 1.4 to correct for the calculation of content, is NMT 6 times the area of the principal peak in the Standard solution.
Unspecified impurities: NMT 0.5%; the peak area of each impurity in the Sample solution is NMT the area of the principal peak in the Standard solution.
Total impurities: NMT 1.0%; the total peak area for all unspecified impurities in the Sample solution is NMT twice the area of the principal peak in the Standard solution.
4.2 Chloride and Sulfate, Chloride〈221〉
Sample: 0.2 g
Control: 0.10 mL of 0.020 N hydrochloric acid
Acceptance criteria: 0.035%; the Sample shows no more chloride than the Control.
4.3 Chloride and Sulfate, Sulfate〈221〉
Sample: 0.25 g
Control: 0.30 mL of 0.020 N sulfuric acid
Acceptance criteria: 0.12%; the Sample shows no more sulfate than the Control.
5 SPECIFIC TESTS
Completeness of Solution 〈641〉
Sample solution: 1 g of Methylparaben Sodium dissolved in water
Acceptance criteria: Meets the requirements
pH 〈791〉
Sample solution: 1 mg/mL
Acceptance criteria: 9.5–10.5
Water Determination, Method I〈921〉: NMT 5.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Methylparaben RS


