Methyl Acrylate, Methyl Methacrylate and Methacrylic Acid (7:3:1) Copolymer 280000 Dispersion
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
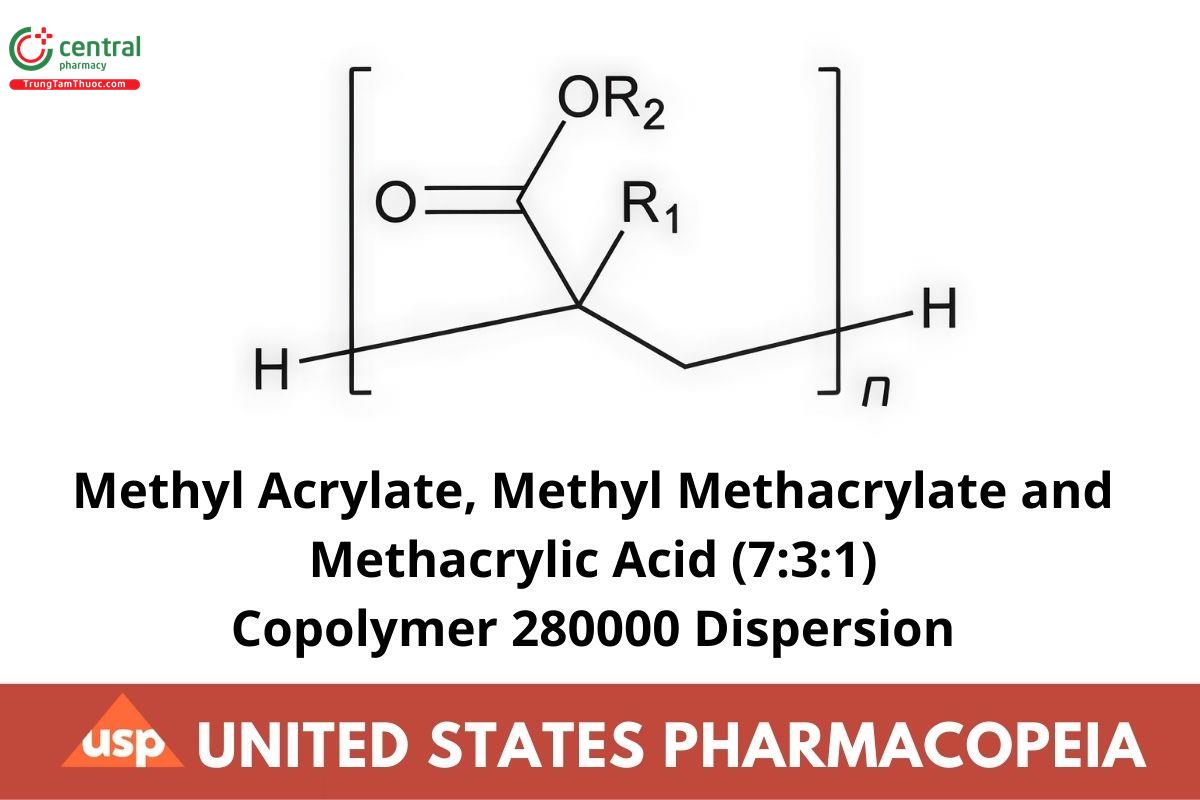
R1 = H; R2 = CH3 or
R1 = CH3; R2 = CH3 or
R1 = CH3; R2 = H
Poly(methyl acrylate-co-methyl methacrylate-co-methacrylic acid) 7:3:1
CAS RN®: 26936-24-3.
1 DEFINITION
Methyl Acrylate, Methyl Methacrylate and Methacrylic Acid (7:3:1) Copolymer 280000 Dispersion is a 30% aqueous dispersion of a synthetic anionic copolymer based on methyl acrylate, methyl methacrylate, and methacrylic acid, with a weight-average molecular weight of approximately 280,000 g/mol. It contains, on the basis of the calculated amount of dry substance in the Dispersion, NLT 9.2% and NMT 12.3% of methacrylic acid units. It may contain suitable surface-active agents.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197F
Analysis: Place 1 drop of Dispersion on a water-resistant crystal disk (silver chloride or KRS-5).1After film formation, dry the crystal disk at 80° for approximately 5 min. Let the crystal disk cool down to room temperature prior to measurement.
Acceptance criteria: The IR absorption spectrum of the Dispersion exhibits maxima corresponding to the same wavelengths as those of a similar preparation of USP Methyl Acrylate, Methyl Methacrylate and Methacrylic Acid (7:3:1) Copolymer 280000 Dispersion RS treated in the same manner.
B. It meets the requirements in the Assay.
3 ASSAY
3.1 PROCEDURE
Sample solution: Dissolve 5 g of the Dispersion in 10 mL of water and add 90 mL of isopropyl alcohol.
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.5 N sodium hydroxide VS
Endpoint detection: Potentiometric
Analysis: Titrate the solution as directed in Titrimetric system. Each milliliter of 0.5 N sodium hydroxide VS is equivalent to 43.045 mg of methacrylic acid (C4H6O2) units.
Calculate, on the dried basis, the percentage of methacrylic acid units in the portion of Dispersion taken:
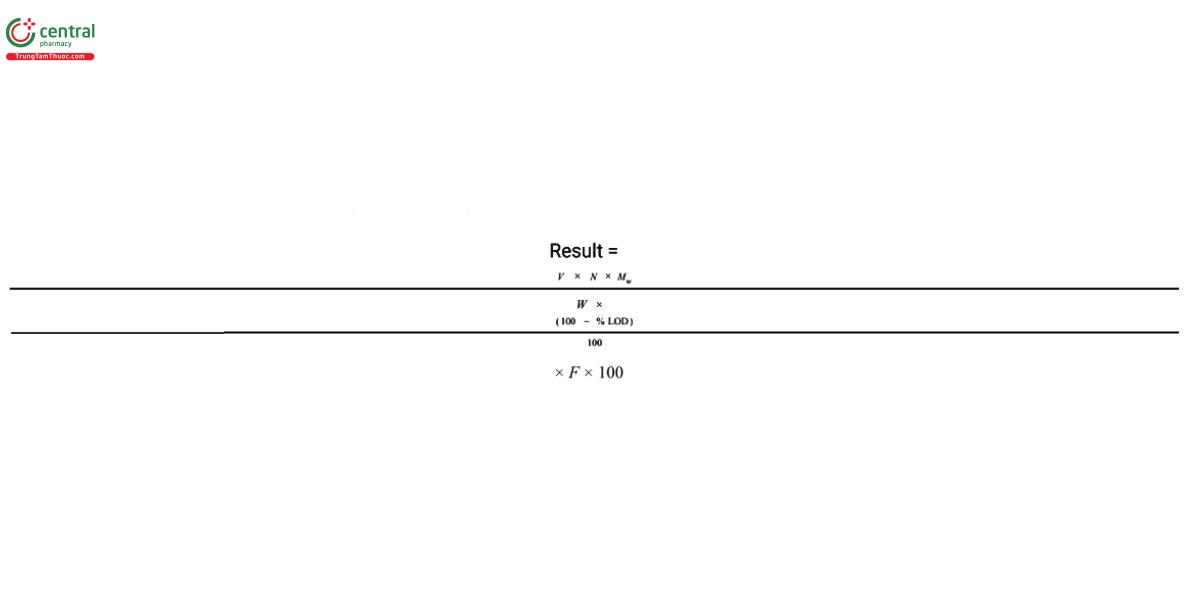
V = volume of Titrant consumed (mL)
N = normality of the Titrant (mol/L)
Mw = molecular weight of methacrylic acid, 86.09 g/mol
W = weight of Dispersion taken (g)
% LOD = the percentage value obtained from the test for Loss on Drying
F = conversion factor, 10-3 L/mL
Acceptance criteria: 9.2%-12.3% based on the calculated amount of dry substance in the Dispersion
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
Sample: 2.0 g of the Dispersion
Analysis: Using mild heating conditions (e.g., steam bath, sand bath) to avoid loss of material, evaporate the Dispersion to dryness prior to ignition.
Acceptance criteria: NMT 0.2% residue is obtained, calculated on the weight of the Dispersion taken
4.2 ORGANIC IMPURITIES
4.2.1 Limit of Monomers
Solution A: Add phosphoric acid dropwise to water to obtain a solution with a pH of 2.0.
Solution B: Methanol and Solution A (70:30) (v/v)
Mobile phase: Acetonitrile and Solution A (10:90) (v/v)
Standard solution: Dissolve approximately 12 mg each of methacrylic acid, methyl acrylate, and methyl methacrylate, each accurately weighed, in 5 mL of isobutyl alcohol, and add acetone to make exactly 50 mL. Dilute 5.0 mL of the solution to 50 mL with acetone. Dilute 20 mL of the solution to 50 mL with acetone. Take 5.0 mL of the solution and mix with 20 mL of Solution B. This solution contains about 0.002 mg/mL each of methacrylic acid, methyl acrylate, and methyl methacrylate:
Sample solution: Transfer 11 g of the Dispersion to a 50-ml volumetric flask, dilute with acetone to volume, and mix. Add 5 mL of this solution dropwise while continuously stirring into a beaker that contains 20.0 mL of Solution B, accurately measured. Remove the precipitated polymer by centrifugation of an aliquot until the solution is clear. 2Use the clear supernatant.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 4.6-mm x 12.5-cm; 7-µm packing 11
Flow rate: 2 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
[NOTE-The relative retention times for methacrylic acid, methyl acrylate, and methyl methacrylate are 1.0, 1.2, and 3.3, respectively.]
Suitability requirements
Resolution: NLT 1.5 between methacrylic acid and methyl acrylate; NLT 8.0 between methyl acrylate and methyl methacrylate
Tailing factor: 0.8-1.5, determined for each analyte
Relative standard deviation: NMT 5.0%, determined for each analyte
4.2.2 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each monomer in the weight of the Dispersion taken:
Result = (rU /rS) × (CU /CS) × 100
rU = peak response of the monomer (methacrylic acid, methyl acrylate, or methyl methacrylate) from the Sample solution
rS = peak response of the monomer (methacrylic acid, methyl acrylate, or methyl methacrylate) from the Standard solution
CU = concentration of the monomer (methacrylic acid, methyl acrylate, or methyl methacrylate) in the Standard solution (mg/mL)
CS = concentration of the Dispersion in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.01% for total monomers, based on the weight of the Dispersion taken
5 SPECIFIC TESTS
5.1 COAGULUM CONTENT
Analysis: Weigh a stainless steel sieve with 90-µm openings or a suitable single-woven wire cloth with a mesh width of 90 µm, and filter 100 g of the Dispersion through it. [NOTE-Suitable single-woven wire cloth mesh meets the requirements set in ISO 9044.] Wash the sieve or the cloth with distilled water until a clear filtrate is obtained, and dry the sieve or the cloth to constant weight at 110°".
Acceptance criteria: The weight of the residue is NMT 1000 mg/100 g (1%).
5.2 LOSS ON DRYING (731)
Analysis: Dry at 110° for 5 h.
Acceptance criteria: 68.5%-71.5%
5.3 PH (791)
2.0-3.5
5.4 MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62)
The total aerobic microbial count is NMT 103 cfu/g, and the total combined molds and yeasts count is NMT 102 cfu/g.
5.5 VISCOSITY
Diluent (ethanol/tris-hydrochloride buffer mixture): Weigh 9.0 g of tris(hydroxymethyl)aminomethane. Transfer it and add 35 mL of 1 N hydrochloric acid into a 1-L volumetric flask. Dissolve in water with stirring and fill up to volume. The pH value of the solution should be 8.3 ± 0.2. Mix the solution and absolute alcohol (Ethanol) using a 1:1 ratio to a homogenized solution by shaking or stirring. [NOTE-The shelf life of the Diluent is 12 weeks.]
Sample solution: Accurately weigh about 350 mg of the sample into a 100-mL volumetric flask. Dissolve it completely in 50 mL of Diluent with shaking for at least 1 h. Fill the graduated flask with Diluent to below the calibration mark, and adjust the temperature to 20° in a water bath for at least 15 min. Fill up to the calibration mark with Diluent.
Blank solution: Prepare as directed in the Sample solution without adding the sample.
Analysis: The determination is carried out with an automatic suspended-level capillary viscometer (Eigure 1).
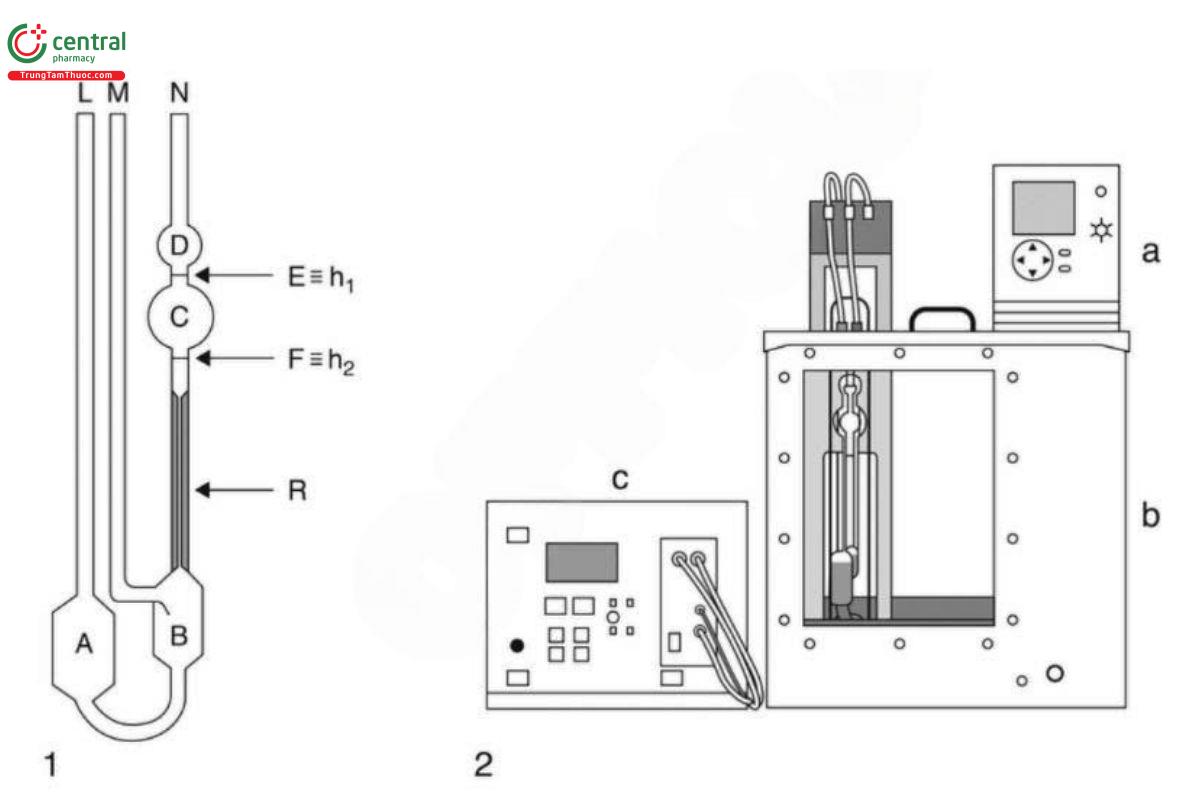
Figures and characters in Figure 1 are defined as follows. 1: specific view of capillary viscometer; 2: automatic suspended-level capillary viscometer, including the temperature control unit (a), heat bath (b), and viscosity measuring unit (c).
Calibrate the viscometer at the test temperature by using fluids of known viscosities of appropriate viscosity standards to determine the viscometer constant, k.
Calculate the viscometer constant, k, in mm2/s2:
k = η/(p x t)
η = known viscosity of the liquid (mPa · s)
p = density of the liquid (g/mL)
t = flow time for the liquid to pass from the upper mark to the lower mark (s)
Using a glass suction filter with a pore diameter of 40-100 µm, filter the Blank solution. [NOTE-Rinse the filter properly with the Blank solution before filtering the solution for the measurement.] Measure the filtered Blank solution at 20". Fill the viscometer through tube (L) with a sufficient quantity of the blank liquid that is appropriate for the viscometer being used or by following the manufacturer's instructions. Carry out the filling with the tube in a vertical position. Fill bulb (A) with the liquid, and also ensure that the level of liquid in bulb (B) is below the exit to the ventilation tube (M). Then place the viscometer into the measurement stand that is located in the temperature-controlled bath stabilized at 20°, and control the temperature to ±0.1°. Maintain the viscometer in a vertical position for a time period of NLT 30 min to allow the sample temperature to reach equilibrium. Prior to the measurement, the liquid to be measured is sucked upward inside the capillary viscometer through two measurement planes (h2 and h1) that are designed as light barriers or thermistor sensors, depending on the viscometer type. The pumping pressure is controlled automatically by a viscosity measuring unit. Design a measuring program/procedure to ensure that the suspended spherical level will form prior to the start of the measurement. Measure the time required for the level of the liquid to drop from mark (E ≡ h1) to mark (F ≡ h2) to a precision of ±0.01 s. Perform the measurement three times for the same filling of the viscometer, with the first measurement considered as a preliminary test (wetting measurement); calculate the mean from the other two flow times.
Repeat the same procedure with the Sample solution.
In the capillary viscometer, the Blank solution (B) or the Sample solution (S) flows through a narrow capillary due to the force of gravity. Calculate viscosity parameters of the Sample solution from the temperature-dependent flow time of the Blank solution, tB, and that of the
Sample solution, tS, in seconds, as follows.
If the flow time is less than the minimum flow time listed in national and international norms per capillary size number, minimize the flow times using the kinetic energy correction (Hagenbach correction) ∆t, in seconds.
Calculate the kinematic viscosity of the Blank solution, νB, and of the Sample solution, νS, in mm2/s:
vB= k x (tB - ∆tB)
vS = k x (tS - ∆tS)
k = viscometer constant (mm²/s²)
∆tB = kinetic energy correction of the Blank solution (s)
∆tS = kinetic energy correction of the Sample solution (s)
The kinetic energy correction can be avoided by choosing a capillary size number with a flow time for the Blank solution or the Sample solution more than the minimum flow time for the used capillary size number listed in national and international norms, because under this circumstance, the kinetic energy correction is typically less than 1%.
Calculate the dynamic viscosity of the Blank solution, ηB, and of the Sample solution, ηS, in mPa · s:
ηB = vB x ρB
ηS = vS x ρS
vB = kinematic viscosity of the Blank solution (mm²/s)
ρB = density of the Blank solution (g/mL)
vS = kinematic viscosity of the Sample solution (mm²/s)
ρS = density of the Sample solution (g/mL)
Incidentally, this also demonstrates the relationship of capillary viscometry (kinematic viscosity, vB or vS) to the rotational viscometry (dynamic viscosity, ηB or ηS).
Calculate the relative viscosity, ηrel, which is larger than 1:
ηrel = ηS/ ηB
At adequately low concentrations, the density of the Blank solution, ρB, can be equated with the density of the Sample solution, ρS, so that
ηrel ≈ vS /vB
Calculate the concentration, c, in g/ml, of the Sample solution:
c = m/V
m = weight of the sample to prepare the Sample solution (g)
V = volume of the Sample solution (mL)
Calculate the specific viscosity, ηspe
ηspe = ηrel - 1
Calculate the reduced viscosity, ned (viscosity number, Staudinger function), in mL/g:
ηred=ηspe/C
For this dispersion measurement, the kinetic energy correction can be avoided. The calculation of reduced viscosity, ηred can be simplified using the following equation:
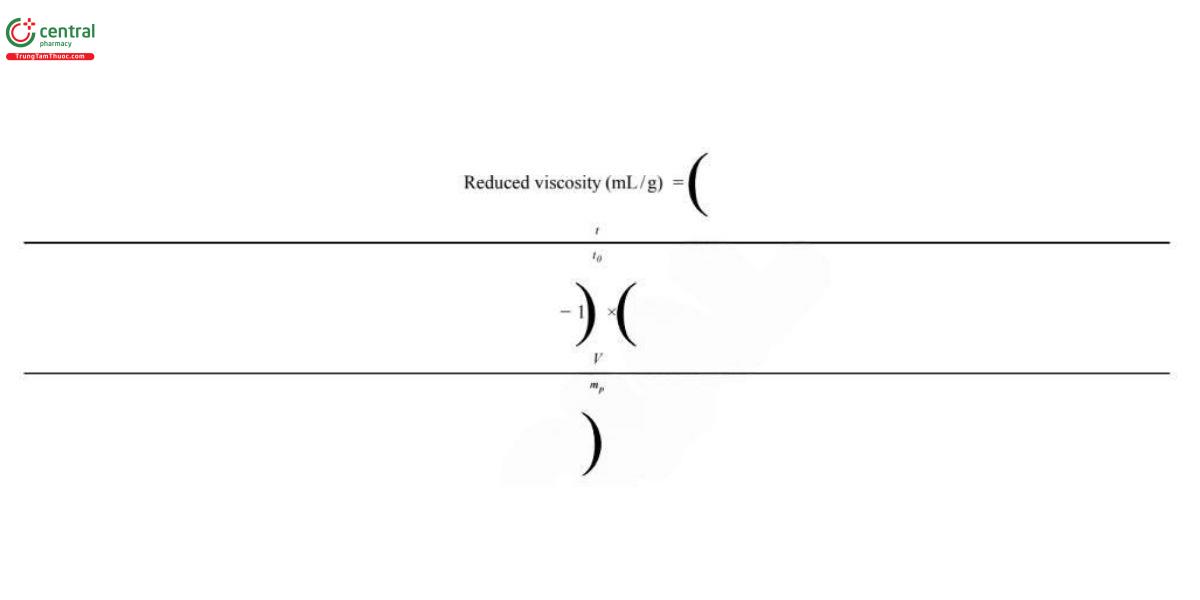
t = mean flow time of the Sample solution (s)
t0 = mean flow time of the Blank solution (s)
V = volume of the Sample solution, 100 mL
mP = weight of the polymer in the Sample solution (g) P
where
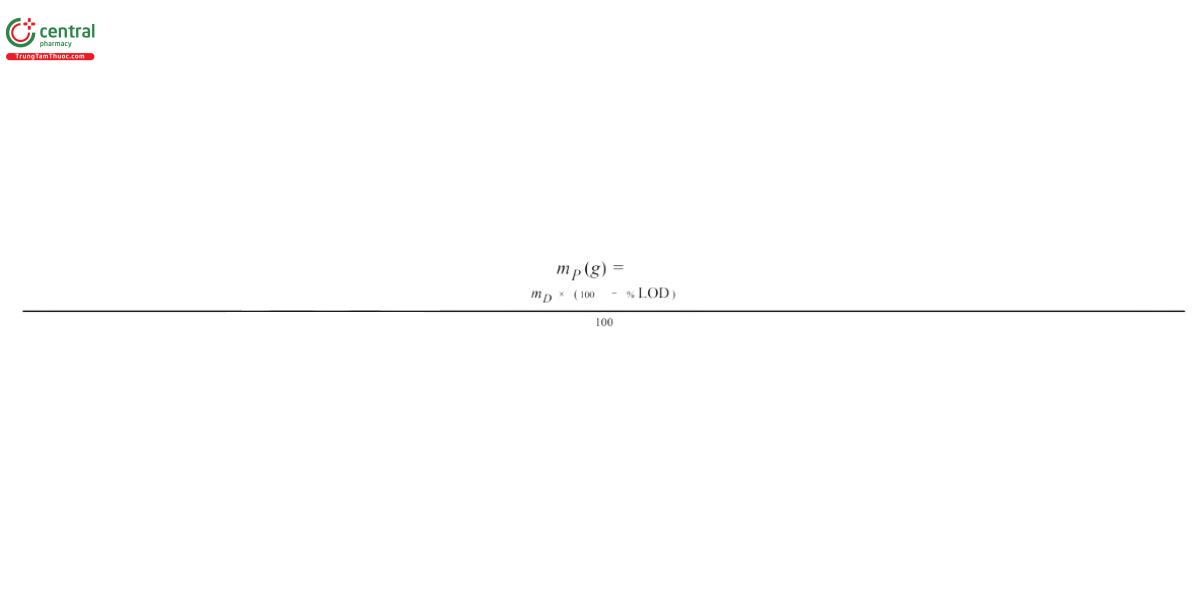
mD = weight of the Dispersion to prepare the Sample solution (g)
% LOD = the percentage value obtained from the test for Loss on Drying
The weight-average molecular weight of the copolymer in the dispersion can be calculated using the following equation: Weight-average molecular weight = (reduced viscosity-2.3936)/0.00038.
Acceptance criteria: 95-130 mL/g for reduced viscosity
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers. Store between 2°-8°. Protect from freezing.
LABELING: The label indicates the name and amount of any substance added as a surface-active agent.
USP Reference Standards 〈11〉
USP Methyl Acrylate, Methyl Methacrylate and Methacrylic Acid (7:3:1) Copolymer 280000 Dispersion RS▲ (NF 1-Aug-2022)
1 KRS-5 consists of 42% thallium(1) bromide and 58% thallium (1) iodine by molecular weight. Suitable disks of silver chloride and of KRS-5 are examples available from www.internationalcrystal.net.
2 Sigma 3-18 centrifuge at 7000 rpm for NLT 5 min has been used in the centrifugation. Equivalent centrifugation conditions are also suitable.


