Methenamine Mandelate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
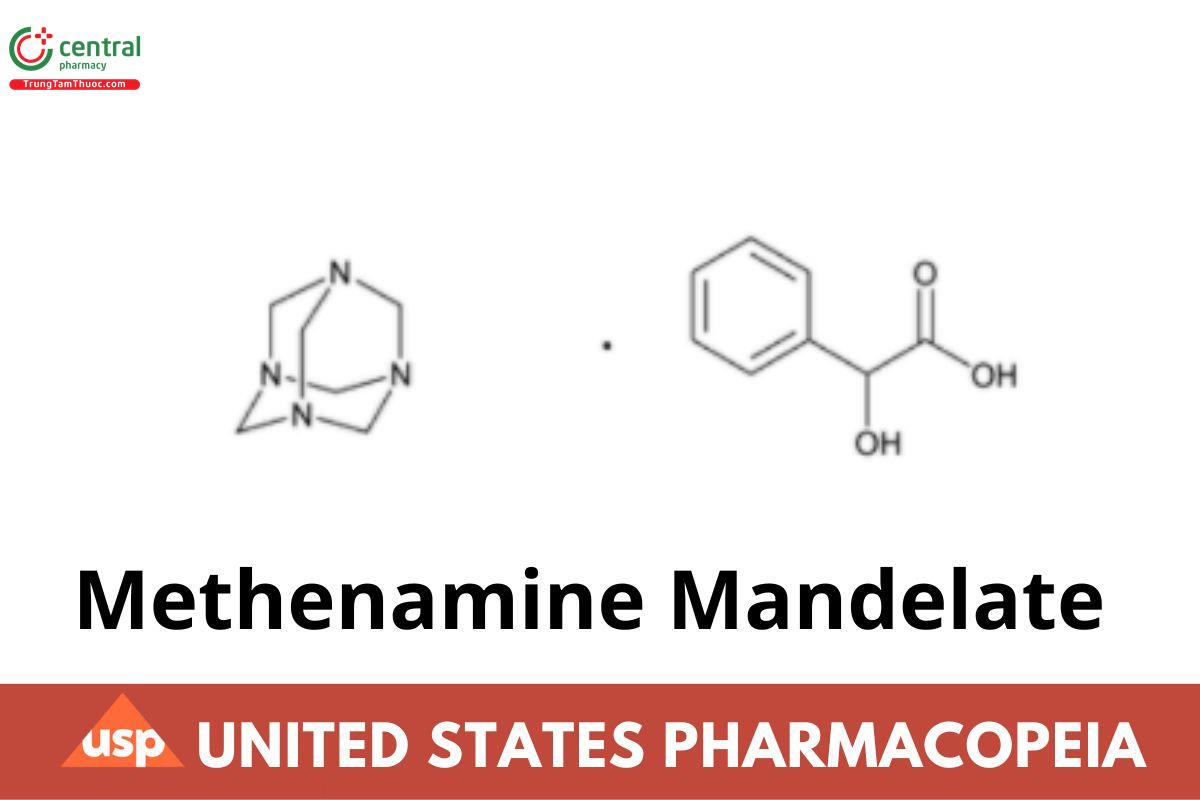
Methenamine Mandelate contains NLT 95.5% and NMT 102.0% of methenamine mandelate (C6H12N4 · C8H8O3), and contains NLT 50.0% and NMT 53.0% of mandelic acid (C8H8O3), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
3 ASSAY
Procedure
Sample solution: Transfer 60 mg of Methenamine Mandelate to a 250-mL conical flask. Add 15 mL of dehydrated alcohol, stir to dissolve, and add 40 mL of chloroform.
Titrimetric system
Mode: Direct titration
Titrant: 0.05 N silver nitrate in dehydrated alcohol prepared as follows. Dissolve by stirring 8.5 g of silver nitrate in 1000 mL of dehydrated alcohol. Transfer 100 mg of sodium chloride, previously dried at 110° for 2 h, to a 100-mL beaker, and dissolve in 50 mL of water. Titrate
with the silver nitrate solution to the potentiometric endpoint, using a silver billet indicator electrode and a silver–silver chloride double-junction reference electrode containing a potassium nitrate salt bridge. Calculate the normality of the titrant.
Endpoint detection: Potentiometric
Analysis: Titrate the Sample solution with Titrant, determining the endpoint potentiometrically, using a silver billet indicator electrode and a silver–silver chloride double-junction reference electrode containing a potassium nitrate salt bridge. Each mL of 0.05 N silver nitrate is equivalent to 7.308 mg of methenamine mandelate (C6H12N4 · C8H8O3).
Acceptance criteria: 95.5%–102.0% on the dried basis
4 OTHER COMPONENTS
Content of Mandelic Acid
Sample solution: Transfer 90 mg to a 250-mL conical flask containing 50 mL of water. When the solution is complete, titrate the magnetically stirred solution.
Titrimetric system
Mode: Direct titration
Titrant: 0.05 N ceric ammonium nitrate VS
Endpoint detection: Potentiometric
Analysis: Titrate the Sample solution with Titrant, determining the endpoint potentiometrically. Each mL of 0.05 N ceric ammonium nitrate is
equivalent to 3.804 mg of mandelic acid (C8H8O3).
Acceptance criteria: 50.0%–53.0% on the dried basis
5 IMPURITIES
Residue on ignition 〈281〉: NMT 0.1%
Chloride and Sulfate, Chloride〈221〉
Sample: 1.0 g
Analysis: Dissolve the Sample in 10 mL of water, and add gradually 500 mg of anhydrous sodium carbonate. Evaporate to dryness, and ignite the residue at a dull-red heat. Add 20 mL of 2 N nitric acid, stir gently, and filter.
Acceptance criteria: 0.01%; the filtrate shows no more chloride than corresponds to 0.15 mL of 0.020 N hydrochloric acid.
Sulfate
Sample: 0.20 g
Analysis: Dissolve the Sample in 10 mL of water. Add 5 drops of 3 N hydrochloric acid and 5 drops of barium chloride TS.
Acceptance criteria: No turbidity appears within 1 min.
6 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry over silica gel for 18 h.
Acceptance criteria: NMT 1.5%
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP Methenamine Mandelate RS


