Methacycline Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
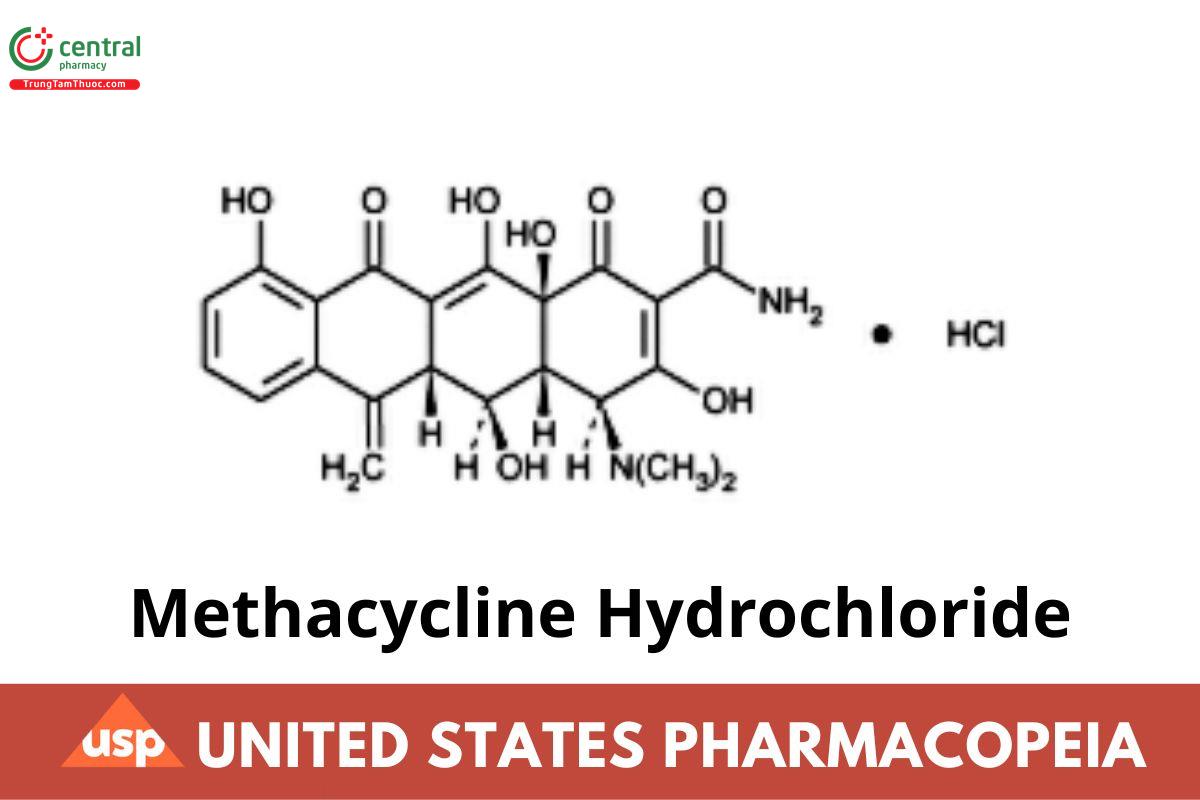
C22H22N2O8. HCl 478.88
2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,lum12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, monohydrochloride, [4S-(4α,4aα,5α,5aα,12aα)]-.
4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride CAS RN®: 3963-95-9; UNII: 9GJ0N7ZAP0.
Methacycline Hydrochloride has a potency equivalent to not less than 832 µg and not more than 970 µg of methacycline (C22H22N2O8) per mg.
Packaging and storage—Preserve in tight, light-resistant containers.
USP Reference standards 〈11〉—
USP Doxycycline Hyclate RS
USP Methacycline Hydrochloride RS
Change to read:
Identication, Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Solution: 20 µg per mL.
Medium: hydrochloric acid in methanol (1 in 1200).
Absorptivity at 345 nm, calculated on the dried basis, is between 88.4% and 96.4% of the USP Methacycline Hydrochloride RS, the potency of the Reference Standard being taken into account.
Crystallinity 〈695〉: meets the requirements.
pH 〈791〉: between 2.0 and 3.0, in a solution containing 10 mg of methacycline per mL.
Water Determination, Method I〈921〉 : not more than 2.0%.
Assay—
Mobile phase—Prepare a mixture of 0.2 M ammonium oxalate, dimethylformamide, and 0.1 M edetate disodium (11:5:4), adjust with tetrabutylammonium hydroxide, 40 percent in water, to a pH of 7.0, and filter. Make adjustments, if necessary (see System Suitability under Chromatography 〈621〉).
System suitability preparation—Prepare a solution of USP Methacycline Hydrochloride RS and USP Doxycycline Hyclate RS in Mobile phase containing about 0.5 mg of each per mL.
Standard preparation—Quantitatively dissolve an accurately weighed quantity of USP Methacycline Hydrochloride RS in Mobile phase to obtain a solution having a known concentration of about 0.5 mg per mL.
Assay preparation—Transfer about 50 mg of Methacycline Hydrochloride, accurately weighed, to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography 〈621〉)—The liquid chromatograph is equipped with a 354-nm detector and a 4.6-mm × 15-cm column that contains 3.5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the System suitability preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.75 for methacycline and 1.0 for doxycycline; and the resolution, R, between methacycline and doxycycline is not less than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in µg, of methacycline (C22H22N2O8) in each mg of Methacycline Hydrochloride taken by the formula:
100(CE/W)(rU/rS)
in which C is the concentration, in mg per mL, of USP Methacycline Hydrochloride RS in the Standard preparation; E is the methacycline content, in µg per mg, of USP Methacycline Hydrochloride RS; W is the quantity, in mg, of Methacycline Hydrochloride taken to prepare the
Assay preparation; and rU and rS are the methacycline peak areas obtained from the Assay preparation and the Standard preparation, respectively.


