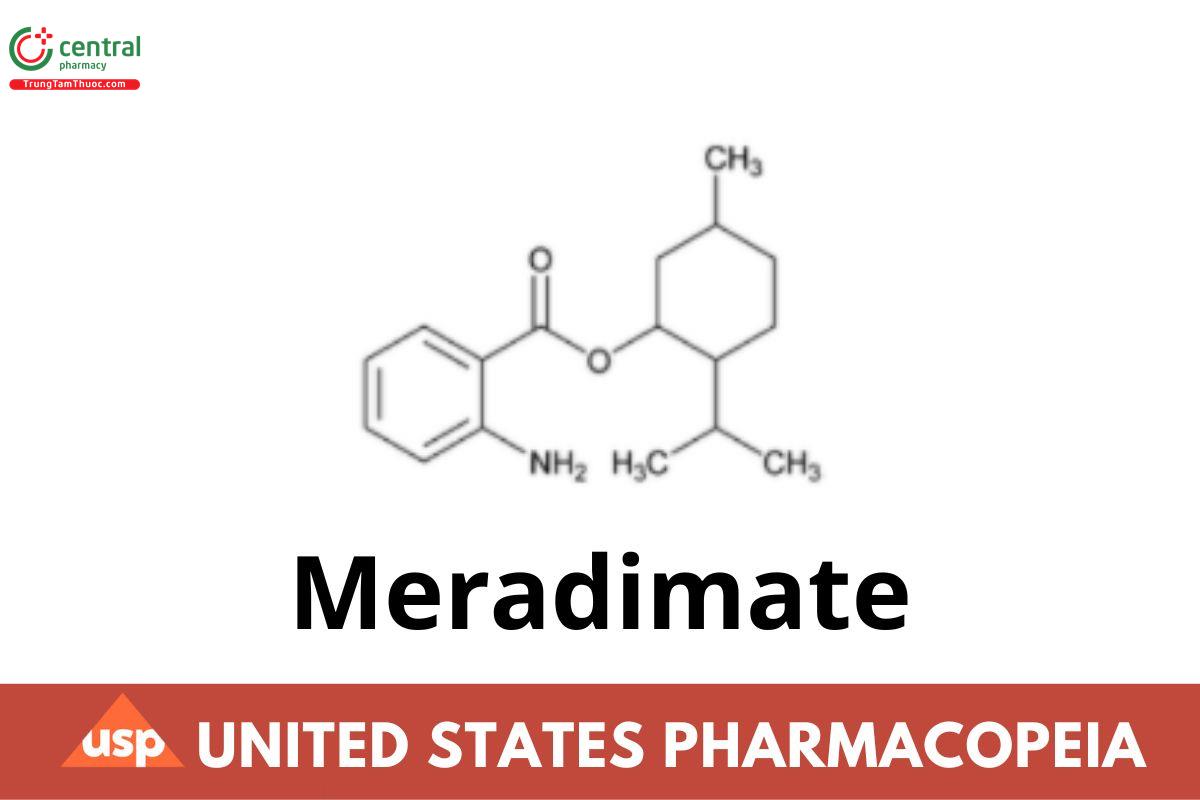
Meradimate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Meradimate contains NLT 95.0% and NMT 105.0% of meradimate (C17H25NO2).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F (CN 1-May-2020)
Change to read:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Sample solution: 5 μg/mL in alcohol
Acceptance criteria: Meets the requirements
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Standard solution: 20.0 mg/mL of USP Meradimate RS in tert-butyl methyl ether
Sample solution: 20 mg/mL of Meradimate in tert-butyl methyl ether
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 25-m; coated with a 0.1-μm film of G1
Temperatures
Injector: 240°
Detector: 260°
Column: See Table 1.
Table 1
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 60 | 8 | 240 | 10 |
Carrier gas: Helium
Flow rate: 6 mL/min
Injection type: Split ratio, 30:1
[Note—The split ratio can be modified to optimize performance.]
Injection volume: 1 μL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of meradimate (C17H25NO2) in the portion of Meradimate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Meradimate RS in the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 95.0%–105.0%
4 IMPURITIES
Organic Impurities
Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Meradimate taken:
Result = (rU/rT) × 100
rU = peak response of each impurity
rT = sum of all the peak responses
Acceptance criteria
Any individual impurity: NMT 0.5%
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
Acidity
Sample: 5.0 mL of Meradimate
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N sodium hydroxide VS
Endpoint detection: Visual
Analysis: Transfer 50 mL of alcohol to a suitable container, add 1 mL of phenolphthalein TS, and add sufficient volume of Titrant to obtain a persistent pink color. Transfer 50 mL of this solution to a suitable container, add the Sample, and titrate with Titrant.
Acceptance criteria: NMT 0.2 mL of Titrant per mL of Meradimate is necessary.
Optical Rotation, Specific Rotation〈781S〉: −4° to +4°
Sample solution: 10 mg/mL in alcohol
Refractive Index 〈831〉: 1.540–1.544 at 20°
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Meradimate RS


