Meprobamate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
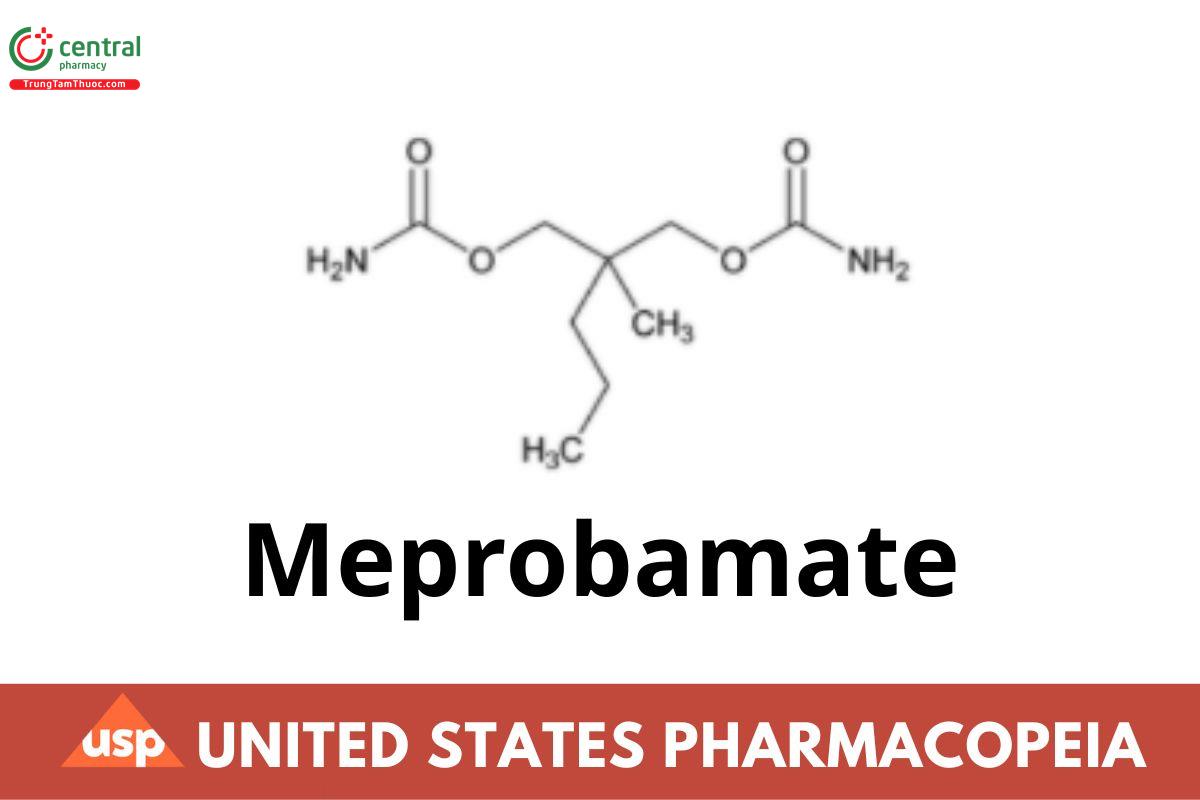
1 DEFINITION
Meprobamate contains NLT 97.0% and NMT 101.0% of meprobamate (C9H18N2O4), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
Sample: 1 mg in 200 mg
Acceptance criteria: The IR absorption spectrum of a potassium bromide dispersion of the Sample, previously dried, exhibits maxima only at the same wavelengths as that of a similar preparation of USP Meprobamate RS. If a difference appears, dissolve portions of both the
Sample and the Reference Standard in acetone at a concentration of 8 mg/mL. Dilute 0.1-mL portions of the acetone solutions with 1 mL of n-heptane, and remove the solvents by evaporation under nitrogen at a temperature of 30°. Dry the residues under vacuum at room temperature for 30 min, and repeat the test on the residues.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: Acetonitrile and water (30:70)
Standard solution: 5 mg/mL of USP Meprobamate RS prepared as follows. Dissolve the Standard first in acetonitrile using 30% of final volume. Sonicate if necessary to dissolve, and cool to room temperature. Dilute with water to volume.
Sample solution: 5 mg/mL of Meprobamate prepared as follows. Dissolve the sample first in acetonitrile using 30% of final volume. Sonicate if necessary to dissolve, and cool to room temperature. Dilute with water to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 4.6-mm × 25-cm; 4-μm packing L1
Flow rate: 1 mL/min
Injection volume: 20 μL
Run time: 2 times the retention time of meprobamate
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of meprobamate (C9H18N2O4) in the portion of Meprobamate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Meprobamate RS in the Standard solution (mg/mL)
CU = concentration of Meprobamate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–101.0% on the dried basis
4 IMPURITIES
Organic Impurities: Procedure 1
Standard solutions: Dissolve USP Meprobamate RS in alcohol, and mix to obtain Standard solution A with a known concentration of 1.0 mg/mL. Dilute quantitatively with alcohol to the obtain the Standard solutions with the compositions given in Table 1.
Table 1
Standard Solution | Dilution | Concentration (mg RS/mL) | Percentage (%, for Comparison with Sample) |
| A | (Undiluted) | 1.0 | 1.0 |
| B | (4 in 5) | 0.8 | 0.8 |
| C | (3 in 5) | 0.6 | 0.6 |
| D | (2 in 5) | 0.4 | 0.4 |
| E | 1 in 5) | 0.2 | 0.2 |
Sample solution: 100 mg/mL of Meprobamate in alcohol
Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: Thin-layer chromatographic plate coated with a 0.25-mm layer of chromatographic silica gel
Application volume: 2 μL
Developing solvent system: Hexane, acetone, and pyridine (70:30:10)
Spray reagent: 5 mg/mL of vanillin in a cooled mixture of sulfuric acid and alcohol (80:20)
Analysis
Samples: Standard solutions and Sample solution
Position the plate in a chromatographic chamber, and develop the chromatograms in the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and air-dry the plate for 15 min. Heat the plate at 100° for 15 min, cool, and spray with Spray reagent. Heat the plate at 110° for 15–20 min, cool, and allow the plate to develop blue-purple spots at room temperature. [Note—Color development requires about 30–60 min.]
Examine the plate, and compare the intensities of any secondary spots of the Sample solution with those of the principal spots of the Standard solutions.
Acceptance criteria: No secondary spot of the Sample solution is larger or more intense than the principal spot of Standard solution A (1.0%), and the sum of the intensities of all secondary spots of the Sample solution corresponds to NMT 2.0%.
Organic Impurities, Procedure 2: Limit of Methyl Carbamate
Standard solution: 1.0 mg/mL of methyl carbamate
Sample solution: Transfer 1.0 g of finely powdered Meprobamate to a beaker, add 5.0 mL of water, and stir to wet the powder completely.
Filter the slurry through a small plug of glass wool in the stem of a glass funnel. Use the clear filtrate.
Mobile phase: Water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 3.9–4.6-mm × 25–30-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 50 μL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The peak response of the Sample solution is not greater than that of the Standard solution, corresponding to NMT 0.5% of methyl carbamate.
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry a sample under vacuum at 60° for 3 h.
Acceptance criteria: NMT 0.5%
Melting Range or Temperature 〈741〉: 103°–107°, but the range between the beginning and end of melting is NMT 2°.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Meprobamate RS


