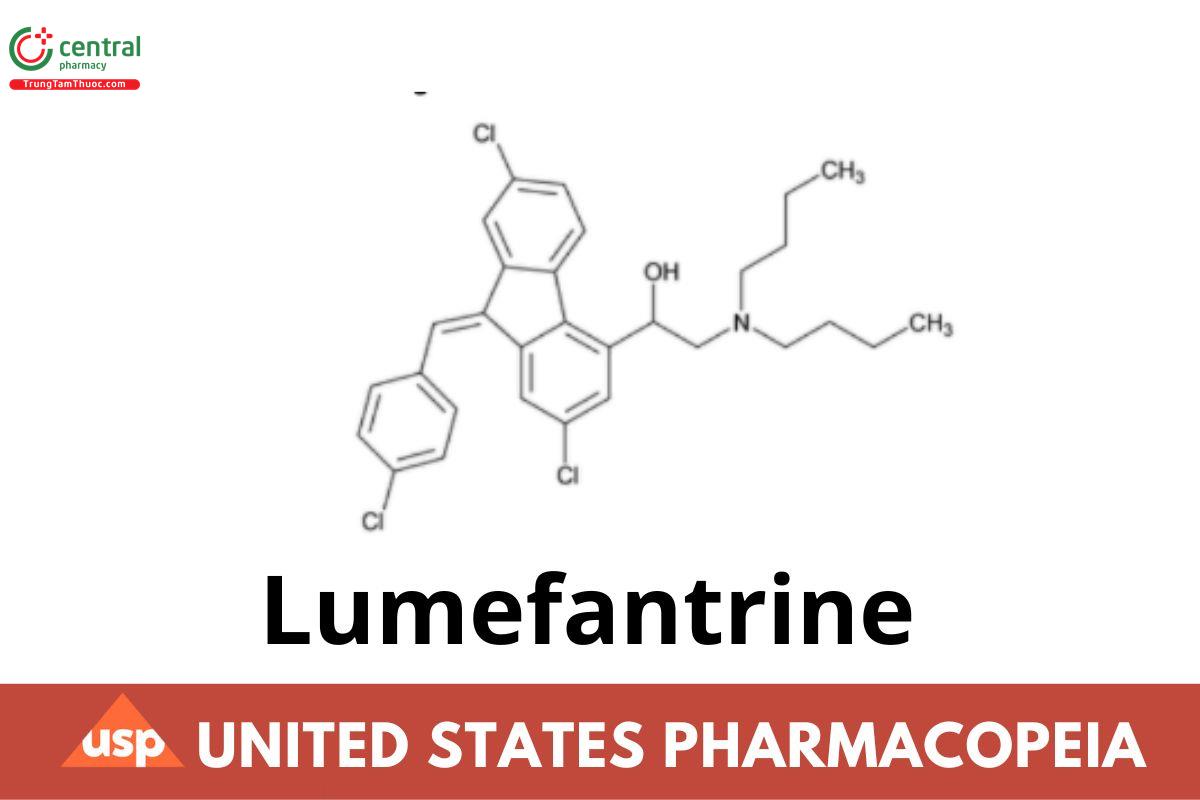
Lumefantrine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Lumefantrine contains NLT 98.0% and NMT 102.0% of lumefantrine (C30H32Cl3NO).
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the lumefantrine peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Buffer: Dissolve 5.65 g of sodium 1-hexanesulfonate and 2.75 g of monobasic sodium phosphate in 900 mL of water. Adjust with phosphoric acid to a pH of 2.3 before dilution with water to a final volume of 1000 mL.
Solution A: Acetonitrile and Buffer (300:700)
Solution B: Acetonitrile and 2-propanol (540:460)
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 65 | 35 |
| 1.2 | 65 | 35 |
| 6.0 | 50 | 50 |
| 6.4 | 30 | 70 |
| 10.0 | 25 | 75 |
| 15.0 | 10 | 90 |
| 15.1 | 65 | 35 |
| 20.0 | 65 | 35 |
System suitability stock solution: 10 μg/mL of USP Lumefantrine Related Compound A RS prepared as follows. Transfer a suitable quantity of USP Lumefantrine Related Compound A RS to a volumetric flask, dissolve in 10% volume dichloromethane, and dilute with acetonitrile to volume.
System suitability solution: 1 mg/mL of USP Lumefantrine RS and 1 μg/mL of USP Lumefantrine Related Compound A RS prepared as follows. Transfer 10 mg of USP Lumefantrine RS to a 10-mL volumetric flask, and dissolve in 1 mL of dichloromethane. Add 1.0 mL of the System suitability stock solution, and dilute with acetonitrile to volume.
Standard solution: 1 mg/mL of USP Lumefantrine RS prepared as follows. Transfer a suitable quantity of USP Lumefantrine RS to a volumetric flask, dissolve in 10% volume of dichloromethane, and dilute with acetonitrile to volume.
Sample solution: 1 mg/mL of Lumefantrine prepared as follows. Transfer a suitable quantity of Lumefantrine to a volumetric flask, dissolve in 10% volume of dichloromethane, and dilute with acetonitrile to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 265 nm
Column: 4.6-mm × 50-mm; 1.8-μm packing L1
Column temperature: Beginning of column, 50°; end of column, 35°
Flow rate: 2.5 mL/min
Injection volume: 2.5 μL
Run time: 20 min
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for lumefantrine related compound A and lumefantrine are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.3 between lumefantrine and lumefantrine related compound A, System suitability solution
Tailing factor: NMT 2.1, Standard solution
Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of lumefantrine (C30H32Cl3NO) in the portion of Lumefantrine taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0%
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Buffer, Solution A, Solution B, Mobile phase, System suitability stock solution, System suitability solution, Sample solution,
Chromatographic system, and System suitability: Proceed as directed in the Assay.
Standard stock solution: 50 μg/mL of USP Lumefantrine RS prepared as follows. Transfer a suitable quantity of USP Lumefantrine RS into a volumetric flask, dissolve in 10% volume of dichloromethane, and dilute with acetonitrile to volume.
Standard solution: 1 μg/mL of USP Lumefantrine RS in acetonitrile from the Standard stock solution
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of desbutyl lumefantrine or any other impurity in the portion of Lumefantrine taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of desbutyl lumefantrine or any other impurity from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
F = relative response factor
Acceptance criteria: See Table 2. Disregard any peak less than 0.05%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Desbutyl lumefantrinea | 0.68 | 1.1 | 0.05 |
| Lumefantrine | 1.0 | - | - |
| Any other individual impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 0.3 |
a (Z)-2-(Butylamino)-1-(2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluoren-4-yl)ethanol.
5 SPECIFIC TESTS
Clarity of Solution
Hydrazine sulfate solution: Transfer 1.0 g of hydrazine sulfate to a 100-mL volumetric flask, and dissolve in and dilute with water to volume.
Allow to stand for 4–6 h before use.
Methenamine solution: Transfer 2.5 g of methenamine to a 100-mL glass-stoppered flask, add 25 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension: Transfer 25.0 mL of Hydrazine sulfate solution to the Methanamine solution in the 100-mL glass-stoppered flask. Allow to stand for 24 h. This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects.
The suspension must not adhere to the glass and must be well mixed before use.
Stock opalescence suspension: Transfer 15.0 mL of the Primary opalescent suspension to a 1000-mL volumetric flask, and dilute with water to volume. This suspension should not be used beyond 24 h after preparation.
Sample solution: Dissolve 1.0 g of Lumefantrine in dichloromethane, and dilute with dichloromethane to 10.0 mL.
Standard suspension: Transfer 5.0 mL of Stock opalescence suspension to a 100-mL volumetric flask, and dilute with water to volume.
Prepare only if the Sample solution is not as clear as water or dichloromethane.
Analysis
Samples: Sample solution, Standard suspension, water, and dichloromethane
Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a at base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of Standard suspension and dichloromethane to separate matching test tubes. Compare the Sample solution, Standard suspension, water, and dichloromethane in diffused daylight, viewing vertically against a black background. The diffusion of light must be such that the Standard suspension can readily be distinguished from dichloromethane. If the Sample solution is as clear as water or dichloromethane, it is not necessary to prepare the
Standard suspension.
Acceptance criteria: The Sample solution shows the same or more clarity than water, dichloromethane, or the Standard suspension.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
USP Reference Standards 〈11〉
USP Lumefantrine RS
USP Lumefantrine Related Compound A RS
(RS,Z)-2-(Dibutylamino)-2-(2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluoren-4-yl)ethanol.
C30H32Cl3NO 528.94


