Lopinavir
If you find any inaccurate information, please let us know by providing your feedback here
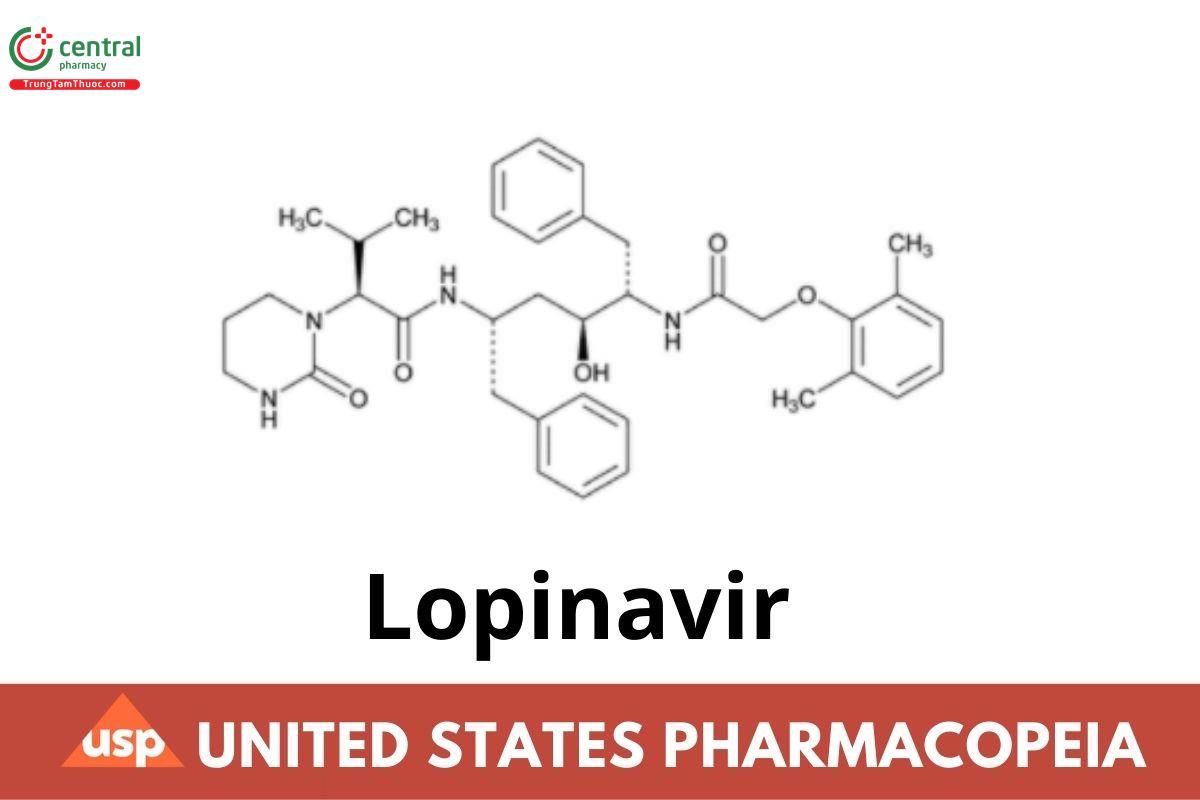
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Lopinavir contains NLT 98.0% and NMT 102.0% of lopinavir (C37H48N4O5), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A (CN 1-May-2020)
B. The retention time of the lopinavir peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Buffer: 2.7 g/L of monobasic potassium phosphate and 0.9 g/L of dibasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 6.0. Pass the solution through a suitable filter of 0.45-μm pore size.
Diluent: Acetonitrile and water (1:1)
Solution A: Acetonitrile and Buffer (9:11)
Mobile phase: Solution A
Standard solution: 0.025 mg/mL of USP Lopinavir RS in Diluent
Sample solution: 0.025 mg/mL of Lopinavir in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 4-μm packing L1
Column temperature: 50°
Flow rate: 1 mL/min
Injection volume: 20 μL
Run time: 60 min
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 8000 theoretical plates
Capacity factor: NLT 15
Tailing factor: 0.8–1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of lopinavir (C37H48N4O5) in the portion of Lopinavir taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Lopinavir RS in the Standard solution (mg/mL)
CU = concentration of Lopinavir in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.2%
Organic Impurities: Procedure 1
[Note—For early-eluting impurities.]
Buffer, Diluent, and Solution A: Prepare as directed in the Assay.
Solution B: Acetonitrile and Buffer (3:1)
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 60 | 100 | 0 |
| 61 | 0 | 100 |
| 81 | 0 | 100 |
| 82 | 100 | 0 |
| 100 | 100 | 0 |
System suitability solution: 0.5 mg/mL of USP Lopinavir System Suitability Mixture RS in Diluent
Standard solution: 0.005 mg/mL of USP Lopinavir RS in Diluent
Sample solution: 0.5 mg/mL of Lopinavir in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 4-μm packing L1
Column temperature: 50°
Flow rate: 1 mL/min
Injection volume: 20 μL
Run time: 100 min
[Note—Data collection is only for the first 60 min. The remaining gradient steps wash out the late-eluting impurities and re-equilibrate the column.]
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times are listed in Table 2.]
Suitability requirements
Resolution: NLT 1.2 between lopinavir N-formylphenoxyacetamide and lopinavir N-acetylphenoxyacetamide, System suitability solution
Capacity factor: NLT 15, Standard solution
Column efficiency: NLT 8000, Standard solution
Tailing factor: 0.8–1.5, Standard solution
Relative standard deviation: NMT 3.0%, Standard solution
Analysis
Samples: Diluent, System suitability solution, Standard solution, and Sample solution
Calculate the percentage of each lopinavir related impurity and unidentified impurity in the portion of Lopinavir taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of lopinavir from the Standard solution
CS = concentration of USP Lopinavir RS in the Standard solution (mg/mL)
CU = concentration of Lopinavir in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Table 2
| Name | Relative Retention | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Lopinavir free aminea | 0.03 | 0.61 | 0.1 |
| Lopinavir N-formylaminoalcoholb | 0.07 | 0.80 | 0.2 |
| Lopinavir divalinatec | 0.10 | 0.65 | 0.1 |
| Sulfolopinavird | 0.13 | 0.76 | 0.1 |
| Lopinavir phenoxyacetamidee | 0.25 | 0.96 | 0.1 |
| Lopinavir N-formylphenoxyacetamidef | 0.59 | 1.3 | 0.1 |
| Lopinavir N-acetylphenoxyacetamideg | 0.62 | 1.2 | 0.1 |
| Lopinavir oxazineh | 0.90 | 1.1 | 0.1 |
| Lopinavir | 1.00 | - | - |
| Isolopinaviri | 1.10 | 0.99 | 0.2 |
| Lopinavir 2,4-phenoxy isomerj | 1.13 | 0.97 | 0.1 |
| Lopinavir d-valine diastereomerk | 1.25 | 1.1 | 0.1 |
| Z-Diacylethenediaminel | 1.28 | 1.4 | 0.1 |
| Lopinavir (2R,4R) diastereomerm | 1.32 | 1.0 | 0.1 |
| Lopinavir (4R) epimern | 1.38 | 0.97 | 0.1 |
| Any other individual impurity | - | 1.0 | 0.1 |
a (S)-N-[(2S,4S,5S)-5-Amino-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
b (S)-N-[(2S,4S,5S)-5-Formamido-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
c (2S,2′S)-N,N′-[(2S,3S,5S)-3-Hydroxy-1,6-diphenylhexane-2,5-diyl]bis{3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide}.
d (2S,3S,5S)-2-[2-(2,6-Dimethylphenoxy)acetamido]-5-{(S)-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamido}-1,6-diphenylhexan-3-yl hydrogen sulfate.
e N-[(2S,3S,5S)-5-Amino-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
f 2-(2,6-Dimethylphenoxy)-N-[(2S,3S,5S)-5-formamido-3-hydroxy-1,6-diphenylhexan-2-yl]acetamide.
g N-[(2S,3S,5S)-5-Acetamido-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
h N-{(S)-1-[(4S,6S)-4-Benzyl-2-oxo-1,3-oxazinan-6-yl]-2-phenylethyl}-2-(2,6-dimethylphenoxy)acetamide.
i (S)-N-{(2S,3S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-3-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
j (S)-N-{(2S,4S,5S)-5-[2-(2,4-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
k (R)-N-{(2S,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
l (Z)-N,N′-(Ethene-1,2-diyl)bis[2-(2,6-dimethylphenoxy)acetamide].
m (S)-N-{(2R,4R,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
n (S)-N-{(2S,4R,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
Organic Impurities: Procedure 2
[Note—For late-eluting impurities.]
Buffer, Diluent, and Solution A: Prepare as directed in the Assay.
Solution B: Acetonitrile and Buffer (3:1)
Mobile phase: Solution A and Solution B (3:7)
System suitability solution: 0.5 mg/mL of USP Lopinavir System Suitability Mixture RS in Diluent
Standard solution: 0.005 mg/mL of USP Lopinavir RS in Diluent
Sample solution: 0.5 mg/mL in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 4-μm packing L1
Column temperature: 50°
Flow rate: 1 mL/min
Injection volume: 20 μL
Run time: 50 min
System suitability
Sample: Standard solution
[Note—The relative retention times are listed in Table 3.]
Suitability requirements
Capacity factor: NLT 1.5
Column efficiency: NLT 3000
Tailing factor: 0.8–1.5
Relative standard deviation: NMT 3.0%
Analysis
Samples: Diluent, System suitability solution, Standard solution, and Sample solution
Calculate the percentage of each lopinavir related impurity and unidentified impurity in the portion of Lopinavir taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of lopinavir from the Standard solution
CS = concentration of USP Lopinavir RS in the Standard solution (mg/mL)
CU = concentration of Lopinavir in the Sample solution (mg/mL)
F = relative response factor (see Table 3)
Table 3
| Name | Relative Retention | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Lopinavir | 1.00 | - | - |
| Lopinavir O-acyla | 1.49 | 0.77 | 0.1 |
| Lopinavir (2R) epimerb | 1.91 | 1.1 | 0.1 |
| Lopinavir diamidec | 4.39 | 1.4 | 0.1 |
| Lopinavir N-acyld | 6.01 | 1.3 | 0.1 |
| Lopinavir O-phenoxyactyle | 7.14 | 1.1 | 0.1 |
Lopinavir amino alcohol ureaf | 8.46 | 1.3 | 0.1 |
| Any other individual impurity | - | 1.0 | 0.1 |
| Total impurities from Procedure 1 and Procedure 2 | - | 1.0 | 0.7g |
a (S)-{(2S,3S,5S)-2-[2-(2,6-Dimethylphenoxy)acetamido]-5-[(S)-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamido]-1,6-diphenylhexan-3-yl} 3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanoate.
b (S)-N-{(2R,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
c N,N′-[(2S,3S,5S)-3-Hydroxy-1,6-diphenylhexane-2,5-diyl]bis[2-(2,6-dimethylphenoxy)acetamide].
d (S)-N-{(2S,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-2-{3-[2-(2,6-dimethylphenoxy)acetyl]-2-oxotetrahydropyrimidin-1(2H)-yl}-3-methylbutanamide.
e (2S,3S,5S)-2-[2-(2,6-Dimethylphenoxy)acetamido]-5-{(S)-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamido}-1,6-diphenylhexan-3-yl 2-(2,6-dimethylphenoxy)acetate.
f N,N′-(2S,2′S,3S,3′S,5S,5′S)-5,5′-Carbonylbis(azanediyl)bis(3-hydroxy-1,6-diphenylhexane-5,2-diyl)bis[2-(2,6-dimethylphenoxy)acetamide].
g Exclude from Organic Impurities, Procedure 2, lopinavir (4R) epimer and any other peak eluting prior to this peak because these are already monitored in Procedure 1.
Acceptance criteria: See Table 2 and Table 3.
5 SPECIFIC TESTS
Water Determination, Method I〈921〉: NMT 4.4%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at room temperature.
USP Reference Standards 〈11〉
USP Lopinavir RS
USP Lopinavir System Suitability Mixture RS
Lopinavir System Suitability Mixture contains lopinavir N-formylphenoxyacetamide, lopinavir N-acetylphenoxyacetamide, and several other minor components.
Lopinavir N-formylphenoxyacetamide is (2-(2,6-dimethylphenoxy)-N-[(2S,3S,5S)-5-formamido-3-hydroxy-1,6-diphenylhexan-2-yl]acetamide.
C29H34N2O4 474.59
Lopinavir N-acetylphenoxyacetamide is (N-[(2S,3S,5S)-5-acetamido-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
C30H36N2O4 488.62


