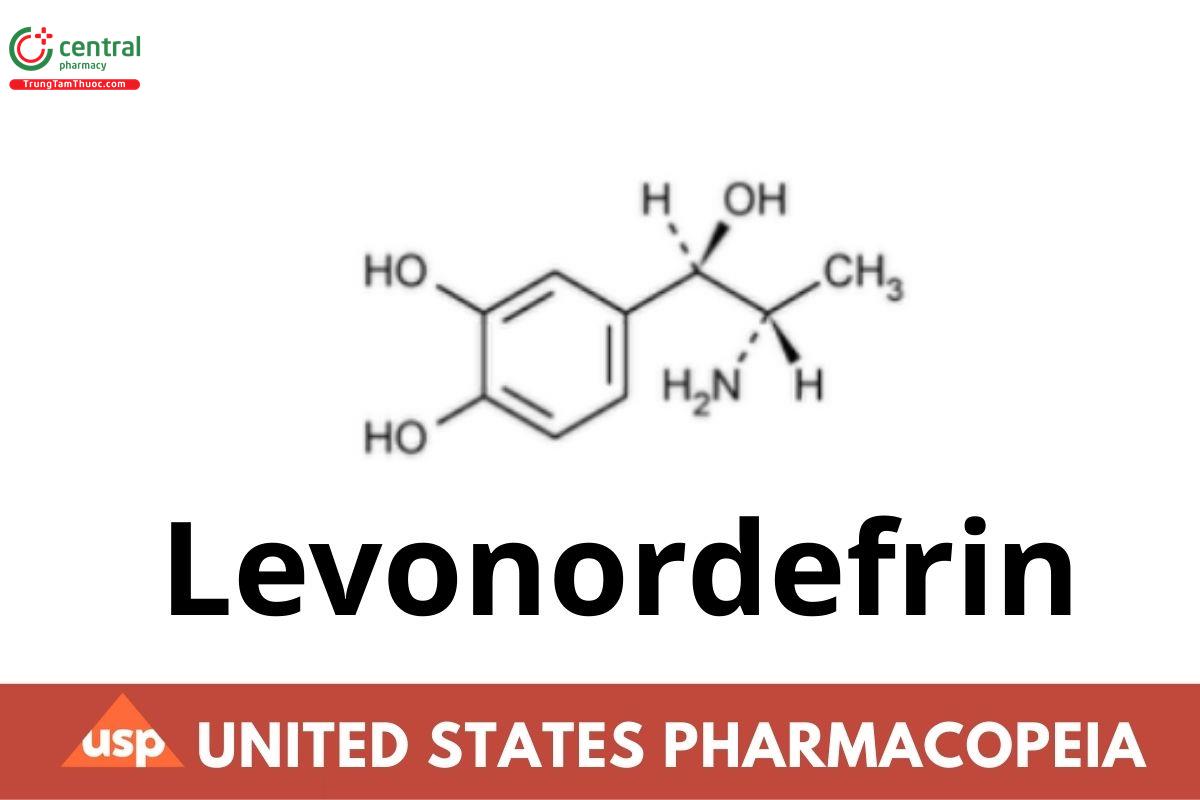
Levonordefrin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
Levonordefrin, dried in vacuum at 60° for 15 hours, contains not less than 98.0 percent and not more than 102.0 percent of C9H13NO3.
Packaging and storage—Preserve in well-closed containers.
USP Reference standards 〈11〉—
USP Levonordefrin RS
Change to read:
1 Identification
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B: Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (ERR 1-May-2020)
Solution: 25 μg per mL.
Medium: 0.1 N hydrochloric acid.
Specific rotation 〈781S〉: between −28° and −31°.
Test solution: 50 mg, previously dried, per mL, in 0.3 N hydrochloric acid.
Loss on drying 〈731〉—Dry it in vacuum at 60° for 15 hours: it loses not more than 1.0% of its weight.
Residue on ignition 〈281〉: not more than 0.2%.
2 Chromatographic purity
Standard solutions—Dissolve an accurately weighed quantity of USP Levonordefrin RS in a mixture of methanol and glacial acetic acid (96:4) to obtain a Standard stock solution having a known concentration of 5 mg per mL. Dilute this solution quantitatively with a mixture of methanol and glacial acetic acid (96:4) to obtain Standard solutions, designated below by letter, having the following compositions:
| Standard solution | Dilution | Concentration (μg RS per mL) | Percentage (%, for comparison with test specimen) |
| A | (1 in 10) | 500 | 1.0 |
| B | (1 in 20) | 250 | 0.5 |
| C | (1 in 50) | 100 | 0.2 |
| D | (1 in 100) | 50 | 0.1 |
Test solution—Dissolve an accurately weighed quantity of Levonordefrin in a mixture of methanol and glacial acetic acid (96:4) to obtain a solution containing 50 mg per mL.
Procedure— Apply separately 5 μL of the Test solution and 5 μL of each Standard solution to a suitable thin-layer chromatographic plate (see
Chromatography 〈621〉) coated with 0.25-mm layer of chromatographic silica gel mixture. Position the plate in a chromatographic chamber, and develop the chromatograms in a solvent system consisting of a mixture of n-butyl alcohol, water, and glacial acetic acid (70:20:10) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate in warm, circulating air. Examine the plate under short-wavelength UV light. Expose the plate to iodine vapors, and examine again. Compare the intensities, observed by both visualizations, of any secondary spots observed in the chromatogram of the Test solution with those of the principal spots in the chromatograms of the Standard solutions: the sum of the intensities of secondary spots obtained from the Test solution corresponds to not more than 1.0% of related compounds, with no single impurity corresponding to more than 0.5%.
3 Assay
Transfer about 350 mg of Levonordefrin, previously dried and accurately weighed, to a smallflask, dissolve in 50 mL of glacial acetic acid, heating, if necessary, add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 18.32 mg of C9H13NO3.


