Levocabastine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C26H29FN2O2 · HCl 456.98
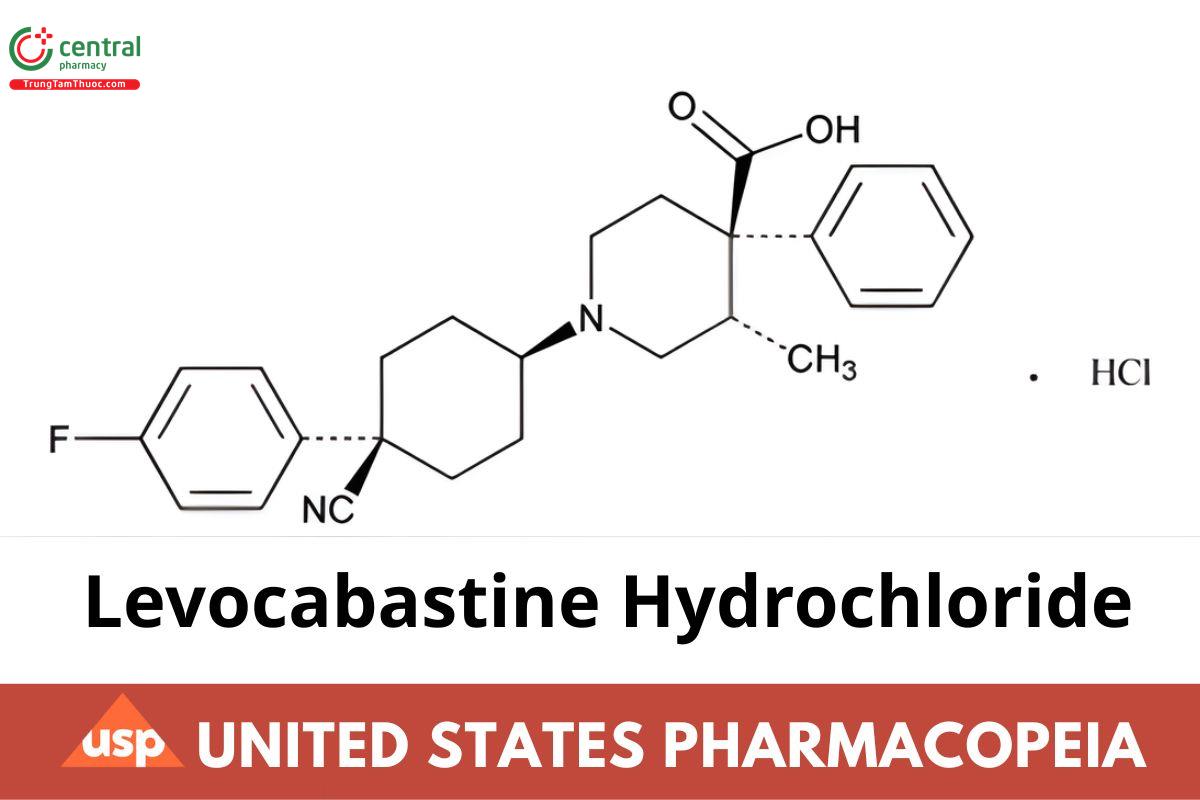
4-Piperidinecarboxylic acid, 1-[4-cyano-4-(4-fluorophen yl)cyclohexyl]-3-methyl-4-phenyl-, monohydrochloride, (–)-[1(cis),3α,4β]-; (–)-trans-1-[cis-4-Cyano-4-(p-fluorophenyl)cyclohexyl]-3-methyl-4-phenylisonipecotic acid monohydrochloride CAS RN®: 79547-78-7; UNII: 124XMA6YEI.
1 DEFINITION
Levocabastine Hydrochloride contains NLT 98.5% and NMT 101.5% of C26H29FN2O2 · HCl, calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. Identification Tests—General, Chloride〈191〉: Meets the requirements
C. Optical Rotation, Specific Rotation〈781S〉: Meets the requirements
3 ASSAY
3.1 Procedure
Sample solution: Dissolve 175 mg of Levocabastine Hydrochloride in 50 mL of alcohol, and add 5.0 mL of 0.01 N hydrochloric acid.
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.1 N sodium hydroxide VS
Endpoint detection: Potentiometric
3.2 Analysis
Sample: Sample solution
The volume of titrant required to titrate Levocabastine Hydrochloride is the difference between the first and third endpoints. Perform a blank determination and make any necessary correction. Each mL of 0.1 N sodium hydroxide VS is equivalent to 22.85 mg of C26H29FN2O2 · HCl.
Acceptance criteria: 98.5%–101.5% on the dried basis
4 IMPURITIES
4.1 Inorganic Impurities
Residue on Ignition 〈281〉: NMT 0.1%, based on a sample weight of about 1.000 g
4.2 Organic Impurities
Procedure
[Note—Prepare solutions immediately before use.]
Diluent: 2 mg/mL of sodium hydroxide in water
Solution A: Dissolve 1.39 g of boric acid in water, and adjust with 1 N sodium hydroxide to a pH of 9.0. Dilute with water to 100 mL.
Run buffer: Dissolve 1.08 g of sodium dodecyl sulfate and 650 mg of hydroxypropyl-β-cyclodextrin in 5 mL of isopropyl alcohol, then dilute with Solution A to 50 mL.
System suitability solution: 12.5 μg/mL of USP Levocabastine Hydrochloride RS and 12.5 μg/mL of USP Levocabastine Related Compound A RS in Diluent Standard solution: Dilute 5.0 mL of the Sample solution with Diluent to 100 mL. Dilute 1.0 mL of this solution with Diluent to 10 mL to obtain a solution containing 12.5 μg/mL of Levocabastine Hydrochloride.
Sample solution: 2.5 mg/mL of Levocabastine Hydrochloride in Diluent
Capillary electrophoresis system
Detector: UV 214 nm
Column: 75-μm × 50-cm uncoated fused-silica capillary column
Column temperature: 50°
Current: See the gradient table below.
| Time (min) | Current (µA) |
|---|---|
| 0 | 0 |
| 0.17 | 75 |
| 15 | 130 |
| 40 | 130 |
| 60 | 200 |
[Note—Before performing the System suitability, equilibrate the capillary column with Diluent for 2 min, then equilibrate with Run buffer for at least 5 min.]
System suitability
Sample: System suitability solution
[Note—The relative migration times for levocabastine and levocabastine related compound A are approximately 1.0 and 1.07, respectively.]
Suitability requirements
Resolution: NLT 4 between levocabastine and levocabastine related compound A
[Note—If necessary, adjust the current gradient to achieve the required resolution.]
Analysis
Samples: Diluent (blank), Standard solution, and Sample solution
Separately inject equal volumes (pressure of 3450 Pa for 5 s) of the Samples, and record the peak responses.
[Note—Disregard any peak originating from the Diluent. Disregard any peak with an area of less than 0.1 times the major peak area of the Standard solution (0.05%).]
Acceptance criteria: The area for any peak in the Sample solution, other than the major peak, is not greater than the major peak area of the Standard solution (0.5%); and the sum of all peak areas in the Sample solution, except for the major peak, is not greater than twice the major peak area of the Standard solution (1.0%).
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation〈781S〉: –102° to −106° at 20°
Sample solution: 10 mg/mL in methanol
Loss on Drying 〈731〉: Dry about 1.000 g of the sample at 105° to constant weight: it loses NMT 0.5% of its weight.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Protect from light.
USP Reference Standards 〈11〉
USP Levocabastine Hydrochloride RS
USP Levocabastine Related Compound A RS


