Levetiracetam
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
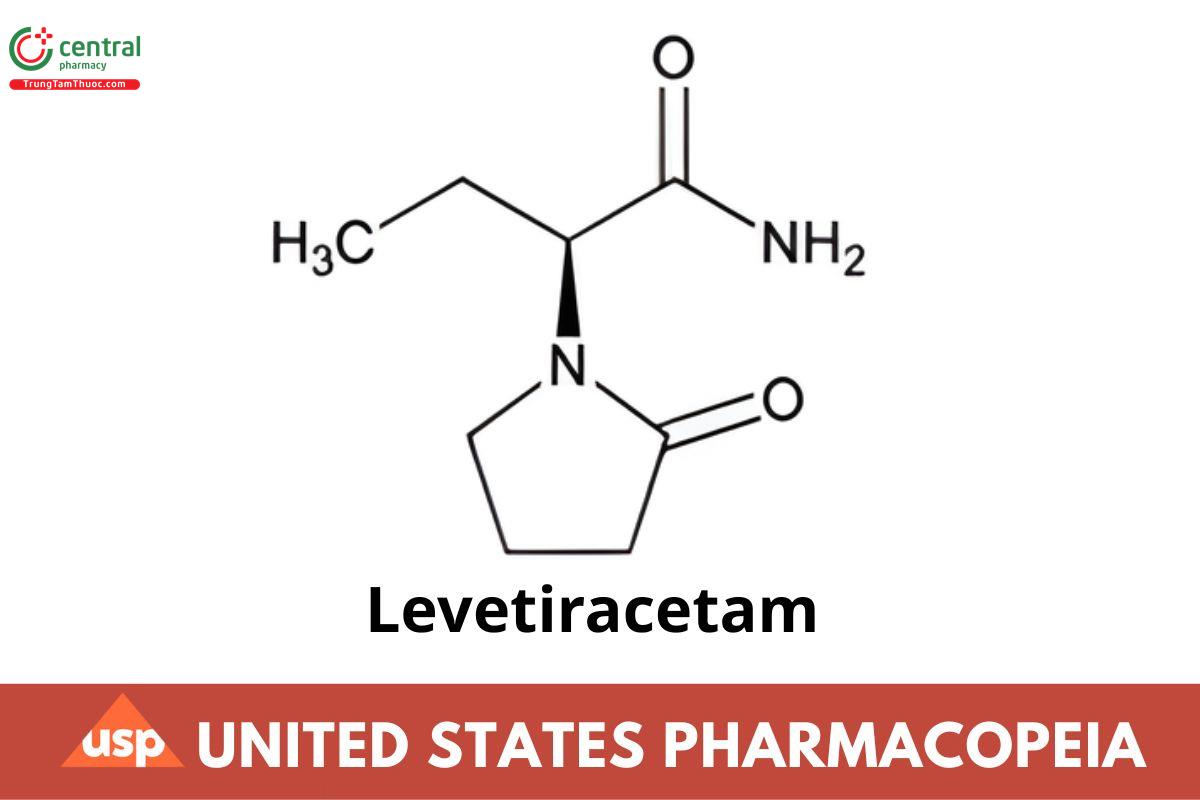
C8H14N2O2 170.21
1-Pyrrolidineacetamide, α-ethyl-2-oxo-, (αS)-;
(−)-(S)-α-Ethyl-2-oxo-1-pyrrolidineacetamide CAS RN®: 102767-28-2; UNII: 44YRR34555.
1 DEFINITION
Levetiracetam contains NLT 98.0% and NMT 102.0% of levetiracetam (C8H14N2O2 ), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The retention time of the major peak of the Identification solution corresponds to that of the levetiracetam S-enantiomer from the System suitability solution, as obtained in the test for Limit of Levetiracetam R-Enantiomer.
3 ASSAY
Procedure
Buffer: 2.7 g/L of monobasic potassium phosphate in water. Adjust with 2% aqueous potassium hydroxide (w/v) to a pH of 5.5.
Solution A: Acetonitrile and Buffer (1:19)
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 3 | 100 | 0 |
| 20 | 71 | 29 |
System suitability solution: 0.2 mg/mL of USP Levetiracetam RS and 0.08 mg/mL of USP Levetiracetam Related Compound A RS in Solution
A. Prepare by first dissolving the required amount of USP Levetiracetam RS in a suitable volumetric flask. Add 10% of the flask volume of 0.1 N potassium hydroxide. Let the mixture react at room temperature for about 15 min, and then neutralize by adding 0.1 N hydrochloric acid at 10% of the flask volume. Add the required amount of USP Levetiracetam Related Compound A RS, sonicate to dissolve, dilute with
Solution A to volume, and mix. [Note—Levetiracetam related compound A is included for peak identification purposes.]
Standard solution: 0.1 mg/mL of USP Levetiracetam RS in Solution A
Sample solution: 0.1 mg/mL of Levetiracetam in Solution A
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 4.6-mm × 15-cm; packing L1
Flow rate: 0.9 mL/min
Injection volume: 10 μL
System suitability
Sample: System suitability solution
[Note—See Table 2 for relative retention times.]
Suitability requirements
Relative standard deviation: NMT 1.0%, for the levetiracetam peak
[Note—If system suitability criteria cannot be met, it is recommended that the column temperature be maintained at 20° to stabilize the
system.]
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of levetiracetam (C8H14N2O2 ) in the portion of Levetiracetam taken:
Result = [(rU/rS)x(CS/CU ) × 100] − F
rU = peak response of levetiracetam from the Sample solution
rS = peak response of levetiracetam from the Standard solution
CS = concentration of USP Levetiracetam RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
F = percentage of levetiracetam R-enantiomer from the test for Limit of Levetiracetam R-Enantiomer
Acceptance criteria: 98.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Limit of Levetiracetam R-Enantiomer
Mobile phase: n-Hexane and dehydrated alcohol (80:20)
System suitability solution: 0.1 mg/mL of USP Levetiracetam Racemic Mixture RS in Mobile phase
Standard solution: 0.05 mg/mL of USP Levetiracetam RS in Mobile phase
Sample solution: 10 mg/mL of Levetiracetam in Mobile phase
Identification solution: 0.05 mg/mL of Levetiracetam from Sample solution in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 10-μm packing L51
Flow rate: 1.0 mL/min
Injection volume: 20 μL
System suitability
Samples: System suitability solution and Identi
cation solution
[Note—The relative retention times for levetiracetam R-enantiomer and levetiracetam S-enantiomer are 0.55 and 1.0, respectively. Use the chromatogram from the Identification solution for Identi
cation test B.]
Suitability requirements
Resolution: NLT 4.0 between the R- and S-enantiomers, System suitability solution. [Note—If a loss of resolution (less than 4.0) is observed, it is recommended that the column temperature be maintained at 25° to stabilize the system.]
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of levetiracetam R-enantiomer in the portion of Levetiracetam taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response of levetiracetam R-enantiomer from the Sample solution
rS = peak response of levetiracetam from the Standard solution
CS = concentration of USP Levetiracetam RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.8%
Limit of Levetiracetam Related Compound B
[Note—Perform this test only if levetiracetam related compound B is a known process impurity.]
Buffer: 1.22 g of sodium 1-decanesulfonate in 1 L of water containing about 1.3 mL of phosphoric acid. Adjust with 20% (w/v) potassium
hydroxide to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (3:17)
System suitability solution: 2 mg/mL of USP Levetiracetam Related Compound B RS in Mobile phase
Standard solution: 0.002 mg/mL of USP Levetiracetam Related Compound B RS in Mobile phase
Sample solution: 2.0 mg/mL of Levetiracetam in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 4.6-mm × 25-cm; packing L1
Flow rate: 1.0 mL/min
Injection volumes
System suitability: 10 μL
Analysis: 50 μL
System suitability
Sample: System suitability solution
[Note—The retention time for levetiracetam related compound B is 9 min.]
Suitability requirements
Tailing factor: NMT 3.0. [Note—If a signi
cant tailing of the levetiracetam related compound B peak is observed (greater than 3.0), it is
recommended that the column temperature be maintained at 27° to stabilize the system.]
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of levetiracetam related compound B in the portion of Levetiracetam taken:
Result = (rU/rS)x(CS/CU) × (Mr1 /Mr2 ) × 100
rU = peak response of levetiracetam related compound B from the Sample solution
rS = peak response of levetiracetam related compound B from the Standard solution
CS = concentration of USP Levetiracetam Related Compound B RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
Mr1 = molecular weight of levetiracetam related compound B free base, 102.1
Mr2 = molecular weight of levetiracetam related compound B, 138.6
Acceptance criteria: NMT 0.10%
[Note—The amount of levetiracetam related compound B measured is to be included in the total impurities in the test for Organic Impurities.]
Organic Impurities
Buffer, Solution A, Solution B, Mobile phase, System suitability solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Standard solution: 0.005 mg/mL of USP Levetiracetam RS in Solution A
Sample solution: 5 mg/mL of Levetiracetam in Solution A
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Levetiracetam taken:
Result = (rU/rS)x(CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of levetiracetam from the Standard solution
CS = concentration of USP Levetiracetam RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
[Note—Disregard any peak with a relative retention time of 0.19 or less.]
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Pyridin-2-ola | 0.37 | 1.0 | 0.025 |
| Levetiracetam acidb | 0.62 | 1.2 | 0.3 |
| Levetiracetam | 1.00 | – | – |
| Levetiracetam related compound Ac | 1.25 | 0.35 | 0.05 |
| Any individual unspecified impurity | – | 1.0 | 0.05 |
| Total impurities | – | – | 0.4 |
a Not included in the total impurities limit.
b (S)-2-(2-Oxopyrrolidin-1-yl)butanoic acid. Included in the total impurities limit.
c (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide. Included in the total impurities limit only if levetiracetam related compound B is a known process impurity.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method Ia: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, and store at room temperature.
USP Reference Standards 〈11〉
USP Levetiracetam RS
USP Levetiracetam Racemic Mixture RS
A 1:1 mixture of:
Levetiracetam S-enantiomer-(2S)-2-(2-oxopyrrolidin-1-yl)butanamide;
Levetiracetam R-enantiomer (2R)-2-(2-oxopyrrolidin-1-yl)butanamide.
USP Levetiracetam Related Compound A RS
(S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide.
C8H15ClN2O2 206.67
USP Levetiracetam Related Compound B RS
(S)-2-Aminobutanamide hydrochloride.
C8H14N2O2 · HCl 138.6


