Levalbuterol Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Change to read:
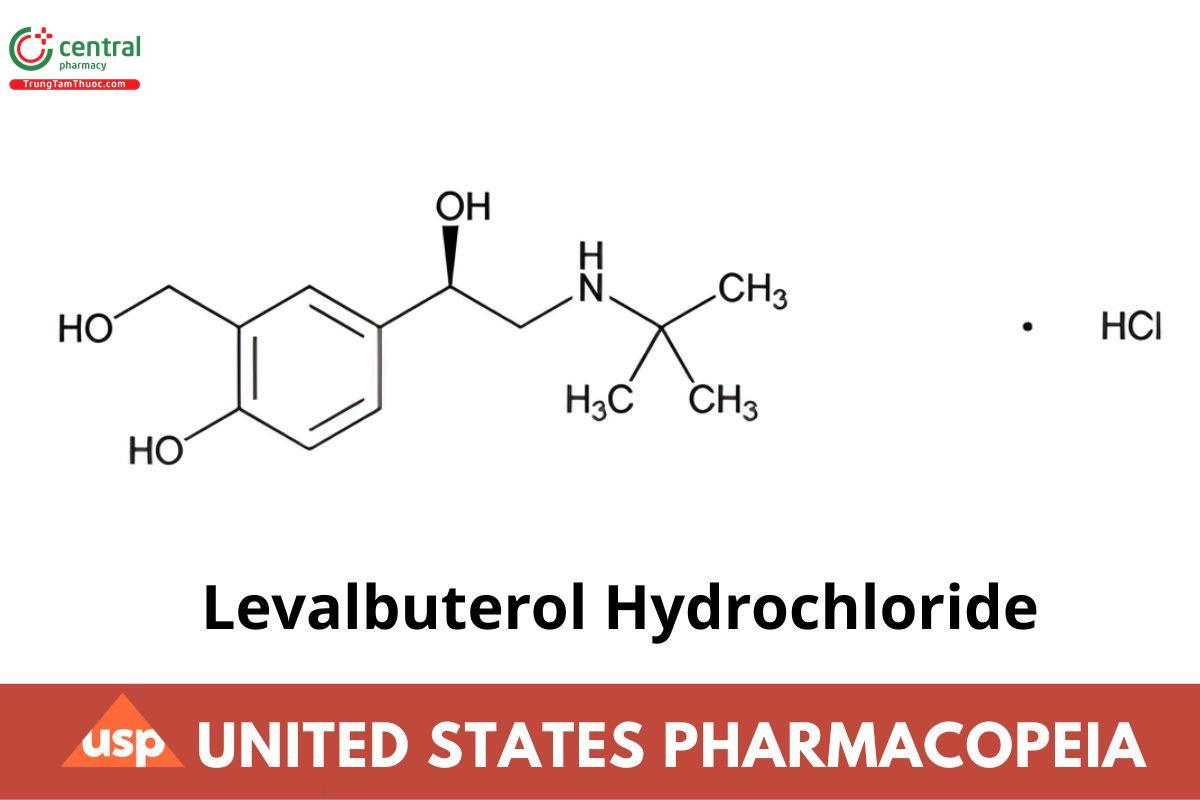
C13H21NO3 · HCl 275.77
Phenol, 4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl), hydrochloride, (R)-;
(R)-α-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene-α,α′-diol hydrochloride; (IRA 1-Nov-2022)
(R)-α1-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene-α,α′-diol hydrochloride CAS RN®: 50293-90-8; UNII: WDQ1526QJM. (IRA 1-Nov-2022)
1 DEFINITION
Levalbuterol Hydrochloride contains NLT 98.0% and NMT 102.0% of levalbuterol hydrochloride (C H NO · HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
Change to read:
B. The retention time of the major peak of the Identification sample solution (IRA 1-Nov-2022) corresponds to that of the levalbuterol peak of the System suitability solution, as obtained in the test for Limit of S-Albuterol.
3 ASSAY
Change to read:
Procedure
Solution A: Dilute 1 mL of phosphoric acid with water to 1 L.
Solution B: Acetonitrile, methanol, and water (35:35:30). To each liter of the solution add 1 mL of phosphoric acid.
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 91.5 | 8.5 |
| 15 | 91.5 | 8.5 |
| 15.01 | 0 | 100 |
| 20 | 0 | 100 |
| 20.01 | 91.5 | 8.5 |
| 30 | 91.5 | 8.5 |
Diluent: Solution A
Standard solution: 100 μg/mL of USP Levalbuterol Hydrochloride RS in Diluent
Sample solution: 100 μg/mL of Levalbuterol Hydrochloride in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 15-cm; 5-μm packing L1
Column temperature: 35°
Flow rate: 1 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.3
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of levalbuterol hydrochloride (C13H21NO3 · HCl) in the portion of Levalbuterol Hydrochloride taken:
Result = (rU/rS)x(CS/CU) × 100
rU = peak response of levalbuterol (IRA 1-Nov-2022) from the Sample solution
rS = peak response of levalbuterol (IRA 1-Nov-2022) from the Standard solution
CS = concentration of USP Levalbuterol Hydrochloride RS in the Standard solution (μg/mL)
CU = concentration of the Sample solution (μg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
Limit of S-Albuterol
Mobile phase: Acetonitrile and methanol (50:50). To each liter of the solution add 3 mL of acetic acid and 1 mL of triethylamine.
Diluent: Mobile phase
System suitability solution: 0.10 mg/mL of USP Levalbuterol Hydrochloride RS and 0.04 mg/mL of USP Albuterol RS in Diluent
Sample solution: 0.8 mg/mL of Levalbuterol Hydrochloride in Diluent
Identification sample solution: (IRA 1-Nov-2022) 0.1 mg/mL of Levalbuterol Hydrochloride (IRA 1-Nov-2022) from the Sample solution in
Diluent (IRA 1-Nov-2022)
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 225 nm
Column: 4.6-mm × 25-cm; 5-μm packing L63
Flow rate: 1 mL/min
Injection volume: 10 μL
Run time: NLT 1.5 times the retention time of levalbuterol
System suitability
Sample: System suitability solution
[Note—The relative retention times for levalbuterol and (S)-albuterol are 1.0 and 1.16, respectively.]
Suitability requirements
Resolution: NLT 2.0 between levalbuterol and (S)-albuterol
Tailing factor: NMT 2.2 for levalbuterol and (S)-albuterol
Analysis
Sample: Sample solution
(IRA 1-Nov-2022)
Calculate the percentage of (S)-albuterol in the portion of Levalbuterol Hydrochloride taken:
Result = (rU/rT) × 100
rU = peak response of (S)-albuterol from the Sample solution
rT = sum of the peak responses for levalbuterol and (S)-albuterol from the Sample solution
Acceptance criteria: NMT 0.2% of (S)-albuterol
Change to read:
Organic Impurities
Solution A, Solution B, Diluent, and Sample solution: Prepare as directed in the Assay.
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 30 | 70 | 30 |
| 50 | 28 | 72 |
| 50.01 | 0 | 100 |
| 55 | 0 | 100 |
| 55.01 | 100 | 0 |
| 70 | 100 | 0 |
System suitability solution: 100 μg/mL of USP Levalbuterol Hydrochloride RS and 0.05 μg/mL each of USP Levalbuterol Related Compound
A RS, USP Albuterol Related Compound A RS, (IRA 1-Nov-2022) USP Levalbuterol Related Compound C RS, and USP Levalbuterol Related
Compound D RS in Diluent
Sensitivity solution: 0.03 μg/mL of USP Levalbuterol Hydrochloride RS in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 15-cm; 5-μm packing L1
Column temperature: 45°
Flow rate: 1 mL/min
Injection volume: 50 μL
System suitability
Samples: System suitability solution and Sensitivity solution
[Note—The relative retention times in Table 3 are provided as information that could aid in peak assignment.]
Table 3 (IRA 1-Nov-2022)
| Name | Relative Retention Time |
|---|---|
| Levalbuterol | 1.0 |
| Levalbuterol related compound A | 1.2 |
| Levalbuterol related compound Ha | 1.3 |
| Albuterol related compound A (IRA 1-Nov-2022) | 1.5 |
| Levalbuterol related compound C | 1.6 |
| Levalbuterol related compound D | 1.7 |
| Levalbuterol related compound Eb | 2.1 |
| Levalbuterol related compound Fc | 3.5 |
Name Relative Retention Time
Levalbuterol 1.0
Levalbuterol related compound A 1.2
Levalbuterol related compound Ha 1.3
Albuterol related compound A (IRA 1-Nov-2022) 1.5
Levalbuterol related compound C 1.6
Levalbuterol related compound D 1.7
Levalbuterol related compound Eb 2.1
Levalbuterol related compound Fc 3.5
a 4-[2-(tert-Butylamino)-1-methoxyethyl]-2-(hydroxymethyl)phenol (IRA 1-Nov-2022) .
b 4-[2-(tert-Butylamino)-1-hydroxyethyl]-2-(ethoxymethyl)phenol; also known as (IRA 1-Nov-2022) α-{[(1,1-Dimethylethyl)amino]methyl}-3-(ethoxymethyl)-4-hydroxy-benzenemethanol.
c 1-[4-(Benzyloxy)-3-(hydroxymethyl)phenyl]-2-(tert-butylamino)ethan-1-ol (IRA 1-Nov-2022) .
Suitability requirements
Resolution: NLT 4.9 between levalbuterol and levalbuterol related compound A; NLT 1.5 between albuterol related compound A (IRA 1-
Nov-2022) and levalbuterol related compound C, System suitability solution
Tailing factor: NMT 4.0 for levalbuterol, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Levalbuterol Hydrochloride taken:
Result = (rU/rT) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
F = relative response factor for each impurity (see Table 4)
Acceptance criteria: See Table 4. The reporting threshold is 0.03%.
Table 4
| Name | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|
| Levalbuterol related compound A | 1.0 | 0.1 |
| Levalbuterol related compound H | 1.0 | 0.15 |
| Albuterol related compound A | 1.0 | 0.10 |
| Levalbuterol related compound C | 1.0 | 0.15 |
| Levalbuterol related compound D | 3.0 | 0.05 |
| Levalbuterol related compound E | 1.0 | 0.1 |
| Levalbuterol related compound F | 1.2 | 0.10 |
| Any unspecified impurity | 1.0 | 0.10 |
| Total impurities | – | 0.5 |
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 10 mg/mL of Levalbuterol Hydrochloride
Acceptance criteria: 4.5–5.5
Water Determination 〈921〉, Method I, Method Ic: NMT 0.3%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store at controlled room temperature.
Change to read:
USP Reference Standards 〈11〉
USP Albuterol RS
USP Albuterol Related Compound A RS
4-{2-[(1,1-Dimethylethyl)amino]-1-hydroxyethyl}-2-methylphenol sulfate (2:1).
(C13H21NO2)2 ∙ H2SO4 544.70 (IRA 1-Nov-2022)
USP Levalbuterol Hydrochloride RS
USP Levalbuterol Related Compound A RS
4-[2-(tert-Butylamino)ethyl]-2-(hydroxymethyl)phenol; Also known as (IRA 1-Nov-2022) 4-(2-tert-Butylamino-ethyl)-2-hydroxymethyl-phenol.
C13H21NO2 223.32 (IRA 1-Nov-2022)
USP Levalbuterol Related Compound C RS
4-[2-(tert-Butylamino)-1-hydroxyethyl]-2-(methoxymethyl)phenol; Also known as (IRA 1-Nov-2022) α-{[(1,1-Dimethylethyl)amino]methyl}-4-hydroxy-3-(methoxymethyl)-benzenemethanol.
C14H23NO3 253.34
USP Levalbuterol Related Compound D RS
5-[2-(tert-Butylamino)-1-hydroxyethyl]-2-hydroxybenzaldehyde sulfate (2:1)
(IRA 1-Nov-2022) ;
Also known as 5-[2-{(1,1-Dimethylethyl)amino}-1-hydroxyethyl]-2-hydroxy-benzaldehyde sulfate(2:1).
(C13H19NO3)2 · H2SO4 572.67


