Leucovorin Calcium
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
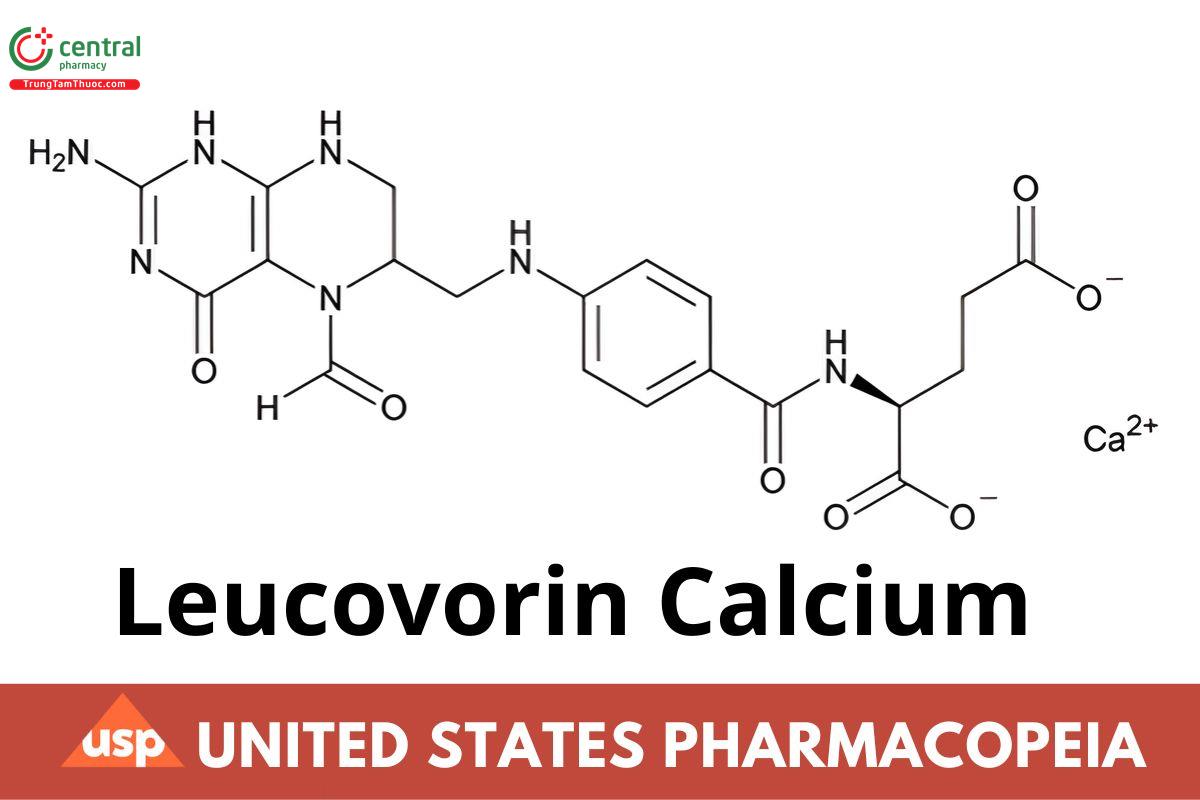
C20H21CaN7O7 511.50
l-Glutamic acid, N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-, calcium salt (1:1);
Calcium [4-({[(RS)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzoyl]-l-glutamate CAS RN®: 1492-18-8; UNII:
RPR1R4C0P4.
1 DEFINITION
Leucovorin Calcium contains NLT 95.0% and NMT 105.0% of leucovorin calcium (C H CaN O ), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197 (CN 1-May-2020) [Note—Methods described in 〈197K〉 or 〈197A〉 may be used.] (USP 1-Aug-2019) Do not dry specimens.
Acceptance criteria: Meets the requirements
Add the following:
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Calcium: Meets the requirements (USP 1-Aug-2019)
Add the following:
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP
1-Aug-2019)
3 ASSAY
Change to read:
Procedure
(USP 1-Aug-2019) Use low-actinic glassware for solutions containing leucovorin calcium, and otherwise protect the solutions from unnecessary exposure to light. Complete the Assay without prolonged interruption.
Solution A: 1.0 M tetrabutylammonium hydroxide in methanol
Solution B: 276 mg/mL of monobasic sodium phosphate monohydrate in water
Mobile phase: Mix 15 mL of Solution A with 835 mL of water. Add 125 mL of acetonitrile, adjust with Solution B to a pH of 7.5 ± 0.1, dilute with water to 1000 mL, and filter. Adjust the concentration of acetonitrile, if necessary.
Diluent: Mix 15 mL of Solution A with 900 mL of water, and adjust with Solution B to a pH of 7.5 ± 0.1. Dilute with water to 1000 mL.
System suitability stock solution: 0.175 mg/mL of USP Folic Acid RS in Diluent
Standard solution: 0.175 mg/mL of USP Leucovorin Calcium RS in Diluent
Sample solution: 0.2 mg/mL of Leucovorin Calcium in Diluent
System suitability solution: System suitability stock solution and Standard solution (1:4)
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4-mm × 30-cm; 5-μm (USP 1-Aug-2019) packing L1
Flow rate: 1.5 mL/min (USP 1-Aug-2019)
Injection volume: 15 μL
Run time: 2.5 times the retention time of the leucovorin peak (USP 1-Aug-2019)
System suitability
Sample: System suitability solution
[Note—The relative retention times for leucovorin and folic acid are 1.0 and about 1.6, respectively.]
Suitability requirements
Resolution: NLT 3.6 between leucovorin
(USP 1-Aug-2019) and folic acid
Relative standard deviation: NMT 2.0% for the leucovorin peak (USP 1-Aug-2019)
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of leucovorin calcium (C20H21CaN7O7) in the portion of Leucovorin Calcium taken:
Result = (rU/rS)x(CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Leucovorin Calcium RS in the Standard solution (mg/mL)
CU = concentration of Leucovorin Calcium in the Sample solution (mg/mL)
Acceptance criteria: 95.0%–105.0% on the anhydrous basis
4 IMPURITIES
Add the following:
Organic Impurities
Use low-actinic glassware for solutions containing leucovorin calcium, and otherwise protect the solutions from unnecessary exposure to light.
Buffer: Dissolve 2.6 mL of tetrabutylammonium hydroxide solution (40% in water) and 2.8 g of disodium hydrogen phosphate in 1 L of water.
Adjust with phosphoric acid to a pH of 7.8.
Mobile phase: Methanol and Buffer (150:850)
System suitability solution: 1.2 mg/mL of USP Leucovorin Calcium RS and 12 μg/mL each of 7,8-dihydrofolic acid and USP Folic Acid RS in water. Sonicate as necessary to dissolve.
Sensitivity solution: 0.4 μg/mL of USP Leucovorin Calcium RS in water
Standard solution: 0.12 mg/mL of USP Leucovorin Calcium RS in water. Sonicate as necessary to dissolve.
Sample solution: 1.1 mg/mL of Leucovorin Calcium in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Temperatures
Autosampler: 10°
Column: 50°
Flow rate: 1 mL/min
Injection volume: 10 μL
Run time: 4 times the retention time of leucovorin
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
Suitability requirements
Resolution: NLT 1.5 between leucovorin and 7,8-dihydrofolic acid, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Leucovorin Calcium taken:
Result = (rU/rS)x(CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of leucovorin from the Standard solution
CS = concentration of USP Leucovorin Calcium RS in the Standard solution (mg/mL)
CU = concentration of Leucovorin Calcium in the Sample solution (mg/mL)
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1. Disregard any impurity peaks less than 0.03%.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| 5-Formyl-5,6,7,8-tetrahydropteroic acida | 0.51 | 1.0 | 0.5 |
| 4-Aminobenzoylglutamic acidb | 0.59 | 1.0 | 1.5 |
| 10-Formyldihydrofolic acidc | 0.76 | 0.47 | 1.0 |
| 5,10-Diformyltetrahydrofolic acidd | 0.85 | 0.49 | 1.5 |
| Leucovorin | 1.0 | 1.0 | – |
| 7,8-Dihydrofolic acide | 1.15 | 1.0 | 0.5 |
| 10-Formylfolic acidf | 2.11 | 0.60 | 0.5 |
| Folic acid | 2.50g | 1.0 | 1.5 |
| Individual, unspecified impurity | – | 1.0 | 0.3 |
| Total impuritiesh | – | – | 2.5 (USP 1-Aug-2019) |
a 4-{[(2-Amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl)methyl]amino}benzoic acid.
b N-(4-Aminobenzoyl)-l-glutamic acid.
c (4-{N-[(2-Amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl]formamido}benzoyl)-l-glutamic acid.
d (4-{N-[(2-Amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl)methyl]formamido}benzoyl)-l-glutamic acid.
e N-(4-{[(2-Amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoyl)-l-glutamic acid.
f (4-{N-[(2-Amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]formamido}benzoyl)-l-glutamic acid.
g RRT may vary from 2.6 to 3.1 and may be compared with the result obtained from the System suitability solution.
h Includes all impurities in Table 1 and 5-(γ-folinoyl)tetrahydrofolate from the test for Limit of 5-(γ-Folinoyl)tetrahydrofolate if the latter is possible from the manufacturing process.
Add the following:
Limit of 5-(γ-Folinoyl)tetrahydrofolate (if present)
The chemical name of 5-(γ-folinoyl)tetrahydrofolate is {4-[({(S)-2-amino-5-[(S)-4-(4-{[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}benzamido)-4-carboxybutanoyl]-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl}methyl)amino]benzoyl}-l-glutamic acid.
Solution A: Acetonitrile
Solution B: Methanol
Solution C: Dissolve 3.25 g of tetrabutylammonium hydroxide solution (40% in water) and 1.36 g of potassium dihydrogen phosphate in 1000 mL of water. Adjust with phosphoric acid to a pH of 7.0.
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) | Solution C (%) |
|---|---|---|---|
| 0 | 1.5 | 4.0 | 94.5 |
| 32 | 1.5 | 15.0 | 83.5 |
| 60 | 1.5 | 35.5 | 63.0 |
| 65 | 1.5 | 4.0 | 94.5 |
| 70 | 1.5 | 4.0 | 94.5 |
Standard solution: 1.0 mg/mL of USP Leucovorin Calcium RS in water
Sample solution: 1.0 mg/mL of Leucovorin Calcium in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 287 nm
Column: 2.1-mm × 10-cm; 3.5-μm packing L1
Column temperature: 40°
Flow rate: 0.3 mL/min
Injection volume: 5 μL
System suitability
Sample: Standard solution
[Note—The relative retention times for leucovorin and 5-(γ-folinoyl)tetrahydrofolate are 1.0 and about 1.7, respectively.]
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Sample: Sample solution
Calculate the percentage of 5-(γ-folinoyl)tetrahydrofolate in the portion of Leucovorin Calcium taken:
Result = (rU/rT) × 100
rU = peak response of 5-(γ-folinoyl)tetrahydrofolate from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria: NMT 0.3%. It is included in the Total impurities in Table 1 if it is possible from the manufacturing process. (USP 1-Aug-
2019)
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 17.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers.
Change to read:
USP Reference Standards 〈11〉
USP Folic Acid RS (USP 1-Aug-2019)
USP Leucovorin Calcium RS


