Leucine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
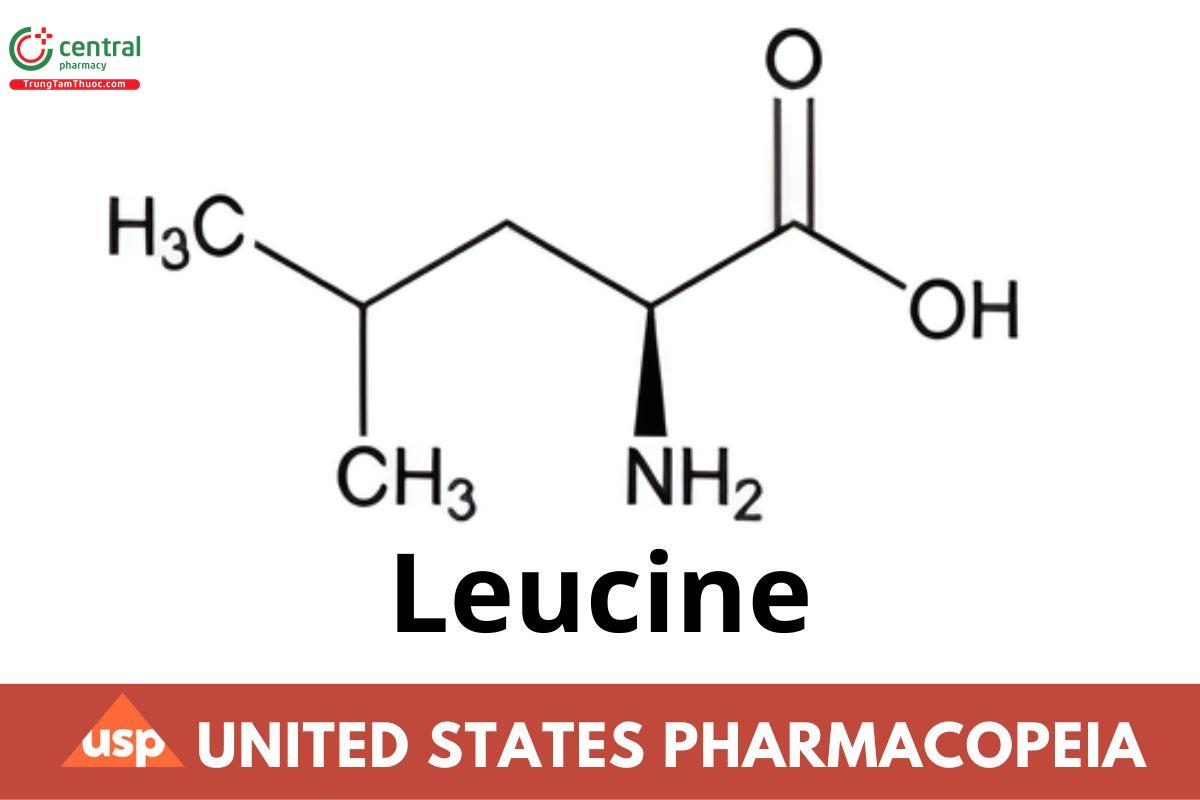
C6H13NO2 131.17
l-Leucine CAS RN®: 61-90-5; UNII: GMW67QNF9C.
1 DEFINITION
Leucine contains NLT 98.5% and NMT 101.5% of l-leucine (C6H13NO2), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
3 ASSAY
Procedure
Sample: 130 mg of Leucine
Blank: Mix 3 mL of formic acid and 50 mL of glacial acetic acid.
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Endpoint detection: Potentiometric
Analysis: Dissolve the Sample in 3 mL of formic acid and 50 mL of glacial acetic acid. Titrate with the Titrant. Perform the blank determination.
Calculate the percentage of leucine (C6H13NO2 ) in the portion of the Sample taken:
Result = {[(VS − VB) × NA × F]/W} × 100
VS = Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
NA = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 131.2 mg/mEq
W = Sample weight (mg)
Acceptance criteria: 98.5%–101.5% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.4%
Chloride and Sulfate 〈221〉, Chloride
Standard solution: 0.50 mL of 0.020 N hydrochloric acid
Sample: 0.73 g of Leucine
Acceptance criteria: NMT 0.05%
Chloride and Sulfate 〈221〉, Sulfate
Standard solution: 0.10 mL of 0.020 N sulfuric acid
Sample: 0.33 g of Leucine
Acceptance criteria: NMT 0.03%
Change to read:
Iron 〈241〉, Procedures, Procedure 1 (CN 1-Jun-2023) : NMT 30 ppm
Related Compounds
System suitability solution: 0.4 mg/mL each of USP l-Leucine RS and USP l-Valine RS in 0.1 N hydrochloric acid
Standard solution: 0.05 mg/mL of USP l-Leucine RS in 0.1 N hydrochloric acid. [Note—This solution has a concentration equivalent to 0.5% of that of the Sample solution.]
Sample solution: 10 mg/mL of Leucine in 0.1 N hydrochloric acid
Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 5 μL
Developing solvent system: Butyl alcohol, glacial acetic acid, and water (3:1:1)
Spray reagent: 2 mg/mL of ninhydrin in a mixture of butyl alcohol and 2 N acetic acid (95:5)
System suitability
Sample: System suitability solution
Suitability requirements: The chromatogram of the System suitability solution exhibits two clearly separated spots.
Analysis
Samples: System suitability solution, Standard solution, and Sample solution
After air-drying the plate, spray with Spray reagent, and heat between 100° and 105° for 15 min. Examine the plate under white light.
Acceptance criteria: Any secondary spot of the Sample solution is not larger or more intense than the principal spot of the Standard solution.
Individual impurities: NMT 0.5%
Total impurities: NMT 2.0%
5 SPECIFIC TESTS
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 40 mg/mL in 6 N hydrochloric acid
Acceptance criteria: +14.9° to +17.3°
pH 〈791〉
Sample solution: 10 mg/mL in water
Acceptance criteria: 5.5–7.0
Loss on Drying 〈731〉
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP l-Leucine RS
USP l-Valine RS


