Ketorolac Tromethamine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
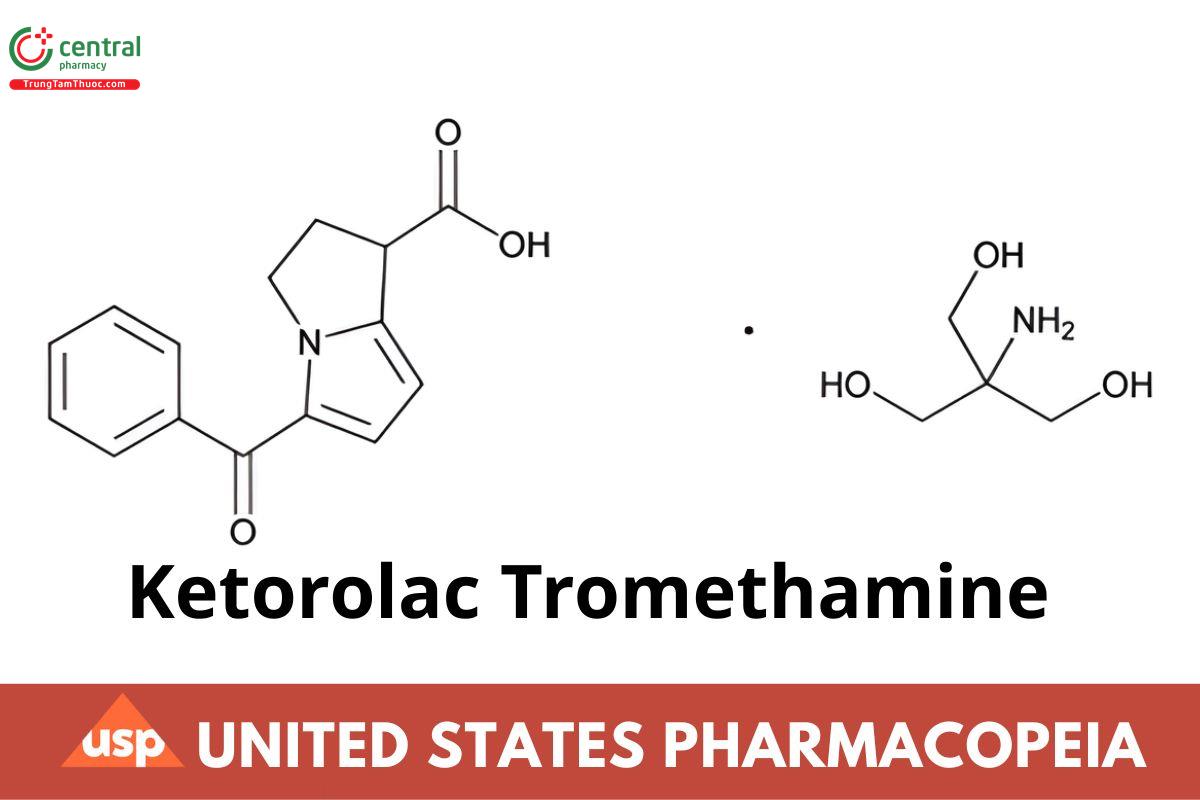
C15H13NO3· C4H11NO3 376.40
1H-Pyrrolizine-1-carboxylic acid, 5-benzoyl-2,3-dihydro, (±)-, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1);
(±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) CAS RN®: 74103-
07-4; UNII: 4EVE5946BQ.
1 DEFINITION
Ketorolac Tromethamine contains NLT 98.5% and NMT 101.5% of ketorolac tromethamine (C15H13NO3· C4H11NO3), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. Thin-Layer Chromatography, Tromethamine Test
Diluent: Dichloromethane and methanol (2:1)
Standard solution: 5 mg/mL of USP Ketorolac Tromethamine RS in Diluent
Sample solution: 5 mg/mL of Ketorolac Tromethamine in Diluent
Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 40 μL
Developing solvent system: Dichloromethane, acetone, and glacial acetic acid (95:5:2)
Spray reagent: Freshly prepared alcoholic solution containing 30 mg/mL of ninhydrin
Analysis
Samples: Standard solution and Sample solution
Develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, and allow the solvent to evaporate. Spray the plate with Spray reagent, and heat the plate at about 150° for 2–5 min.
Acceptance criteria: Yellow spots with pink to purple borders develop on the plate in the areas where the Standard solution and the Sample solution were applied.
3 ASSAY
Procedure
Protect all the solutions from light.
Buffer: 5.75 g/L of monobasic ammonium phosphate. Adjust with phosphoric acid to a pH of 3.0.
Mobile phase: Tetrahydrofuran and Buffer (30:70)
Diluent: Tetrahydrofuran and water (30:70)
System suitability solution: In a 250-mL separator, mix 100 mL of water, 100 mL of dichloromethane, 30 mg of USP Ketorolac Tromethamine RS, and 1 mL of 1 N hydrochloric acid. Insert the stopper, shake, and allow the layers to separate. Transfer the lower dichloromethane layer to a stoppered borosilicate glass flask, and discard the upper layer. Expose the dichloromethane solution to direct sunlight for 10–15 min.
Transfer 1.0 mL of the solution to a vial, evaporate in a current of air or in a stream of nitrogen to dryness, add 1.0 mL of Diluent, and swirl to dissolve. [Note—This solution may be stored under refrigeration and used as long as the chromatogram obtained as directed for Analysis is suitable for identifying the peaks due to the ketorolac 1-keto analog and ketorolac 1-hydroxy analog, and for the measurement of the resolution between the ketorolac 1-keto analog and ketorolac.]
Standard solution: 0.4 mg/mL of USP Ketorolac Tromethamine RS in Diluent
Sample solution: 0.4 mg/mL of Ketorolac Tromethamine in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 313 nm
Column: 4.6-mm × 25-cm; 5-μm packing L7
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for the ketorolac 1-hydroxy analog, the ketorolac 1-keto analog, and ketorolac are about 0.63, 0.89, and 1.0, respectively. Make adjustments, if necessary, to achieve a retention time for ketorolac of about 8–12 min.]
Suitability requirements
Resolution: NLT 1.5 between ketorolac 1-keto analog and ketorolac, System suitability solution
Column efficiency: NLT 5500 theoretical plates, Standard solution
Relative standard deviation: NMT 1.5%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ketorolac tromethamine (C15H13NO3· C4H11NO3) in the portion of Ketorolac Tromethamine taken:
Result = (rU/rS)x(CS/CU) × 100
rU = peak area from the Sample solution
rS = peak area from the Standard solution
CS = concentration of USP Ketorolac Tromethamine RS in the Standard solution (mg/mL)
CU = concentration of Ketorolac Tromethamine in the Sample solution (mg/mL)
Acceptance criteria: 98.5%–101.5% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
Organic Impurities
Mobile phase, Diluent, System suitability solution, Standard solution,
(ERR 1-Jul-2021) Sample solution, and System suitability: (ERR 1-
Jul-2021) Proceed as directed in the Assay.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 313 nm
Column: 4.6-mm × 25-cm; 5-μm packing L7
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 μL
Run time: 3 times the retention time of ketorolac
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Ketorolac Tromethamine taken:
Result = (rU/rT) × F × 100
rU = peak response of each individual impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Impurity having a 0.54 relative retention time | 0.54 | 2.2 | 0.5 |
| Ketorolac 1-hydroxy analog | 0.63 | 0.67 | 0.1 |
| Impurity having a 0.66 relative retention time | 0.66 | 0.91 | 0.5 |
| Ketorolac 1-keto analog | 0.89 | 0.52 | 0.1 |
| Individual unspecified impurity | – | 1.0 | 0.5 |
| Ketorolac tromethamine | 1.0 | 1.0 | – |
| Total impurities | – | – | 1.0 |
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 10 mg/mL
Acceptance criteria: 5.7–6.7
Loss on Drying 〈731〉
Analysis: Dry under vacuum at 60° for 3 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store at 25°, excursions permitted between 15° and 30°.
USP Reference Standards 〈11〉
USP Ketorolac Tromethamine RS


