Isotretinoin
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
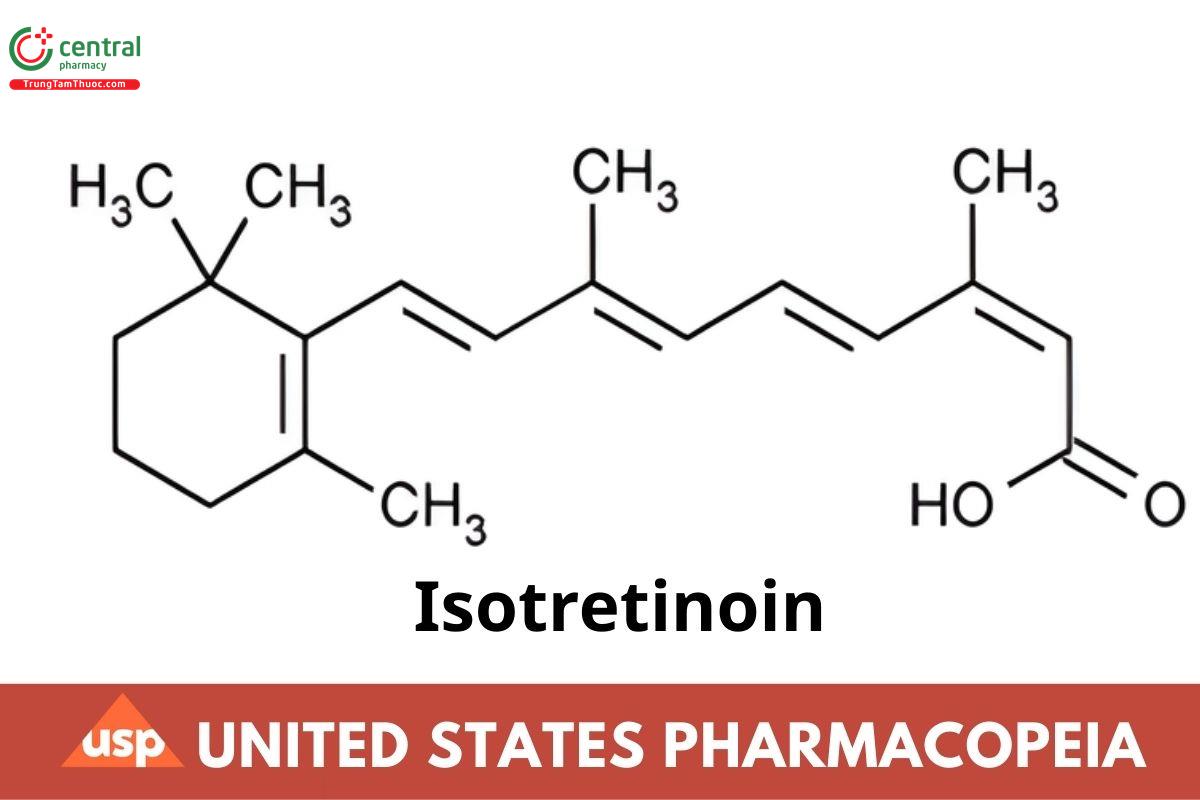
C20H28O2 300.44
Retinoic acid, 13-cis-;
3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)2-cis-4-trans-6-trans-8-trans-nonatetraenoic acid CAS RN®: 4759-48-2; UNII: EH28UP18IF.
1 DEFINITION
Isotretinoin contains NLT 98.0% and NMT 102.0% of isotretinoin (C20H28O2), calculated on the dried basis.
[Caution—Isotretinoin is teratogenic. Avoid inhalation and skin contact.]
[Note—Avoid exposure to strong light, and use low-actinic glassware in the performance of the following procedures.]
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or (USP 1-Dec-2024) 197M
Delete the following:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy (USP 1-Dec-2024)
Add the following:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP 1-Dec-2024)
3 ASSAY
Change to read:
Procedure
Mobile phase: Methanol, glacial acetic acid, and water (77: 0.5: 22.5)
Diluent A: 2 g/L of butylated hydroxytoluene in tetrahydrofuran
Diluent B: Acetonitrile
System suitability solution: Transfer 2 mg of USP Isotretinoin RS into a 100-mL clear glass volumetric flask. Dissolve in 10 mL of Diluent A, and dilute with Diluent B to volume. Expose the flask to ultraviolet light for about 60 min. Protect the solution from light to avoid further degradation of isotretinoin.
[Note—Use of a 366 nm UV lamp as the ultraviolet light source may be suitable.]
[Note—The System suitability solution will contain, in addition to isotretinoin, the degradation products of 2Z,4Z-retinoic acid and 2Z,6Z- retinoic acid. Other degradation products may be present.]
Standard stock solution: 1.0 mg/mL of USP Isotretinoin RS prepared as follows. Transfer an appropriate amount of USP Isotretinoin RS into a suitable volumetric flask. Dissolve in 10% of the flask volume of Diluent A, and dilute with Diluent B to volume. Use the solution immediately to prepare the Standard solution.
Standard solution: 0.1 mg/mL of USP Isotretinoin RS prepared from the Standard stock solution in Diluent B. Store the solution in a refrigerator and inject within 16 h after preparation.
Sample stock solution: 1.0 mg/mL of Isotretinoin prepared as follows. Transfer an appropriate amount of Isotretinoin into a suitable volumetric flask. Dissolve in 10% of the flask volume of Diluent A, and dilute with Diluent B to volume. Use the solution immediately to prepare the Sample solution.
Sample solution: 0.1 mg/mL of Isotretinoin prepared from the Sample stock solution in Diluent B. Store the solution in a refrigerator and inject within 16 h after preparation.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 355 nm
Column: 4.6-mm × 15-cm; 3-μm packing L1
Temperatures
Autosampler: 5o
Column: 30o
Flow rate: 1 mL/min
Injection volume: 20 μL
Run time: NLT 1.5 times the retention time of isotretinoin
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for 2Z,4Z-retinoic acid, isotretinoin, and 2Z,6Z-retinoic acid are 0.98, 1.00, and 1.05, respectively.]
Suitability requirements
Peak-to-valley ratio: NLT 4.0 for 2Z,4Z-retinoic acid and isotretinoin, System suitability solution
Resolution: NLT 1.5 between isotretinoin and 2Z,6Z-retinoic acid, System suitability solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of isotretinoin (C20H28O2) in the portion of Isotretinoin taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of isotretinoin from the Sample solution
rS = peak response of isotretinoin from the Standard solution
CS = concentration of USP Isotretinoin RS in the Standard solution (mg/mL)
CU = concentration of Isotretinoin in the Sample solution (mg/mL) (USP 1-Dec-2024)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Delete the following:
Limit of Tretinoin (USP 1-Dec-2024)
Add the following:
Organic Impurities
Diluent A, Diluent B, and System suitability solution: Prepare as directed in the Assay.
Solution A: 0.5% (v/v) glacial acetic acid in water
Solution B: Acetonitrile, methanol, and Solution A (37:30:33)
Solution C: Methanol and Solution A (77:23)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution B (%) | Solution C (%) |
| 0 | 100 | 0 |
| 20 | 100 | 0 |
| 25 | 0 | 100 |
| 60 | 0 | 100 |
| 61 | 100 | 0 |
| 80 | 100 | 0 |
Standard stock solution: 500 μg/mL each of USP Isotretinoin RS and USP Tretinoin RS prepared as follows. Transfer appropriate quantities of USP Isotretinoin RS and USP Tretinoin RS to a suitable volumetric flask. Dissolve in 10% of the flask volume of Diluent A, and dilute with Diluent B to volume. Store the solution at 8o and use within 14 h after preparation.
Standard solution: 0.5 μg/mL each of USP Isotretinoin RS and USP Tretinoin RS from the Standard stock solution in Diluent B. Store the solution at 8o and inject within 14 h after preparation.
Sensitivity solution: 0.25 μg/mL of USP Isotretinoin RS from the Standard solution in Diluent B. Store the solution at 8o and inject within 14 h after preparation.
Sample solution: 500 μg/mL of Isotretinoin prepared as follows. Transfer an appropriate amount of Isotretinoin to a suitable volumetric flask.
Dissolve in 10% of the flask volume of Diluent A, and dilute with Diluent B to volume. Store the solution at 8o and inject within 14 h after preparation.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 310 and 355 nm
Column: 4.6-mm × 15-cm; 3-μm packing L1
Autosampler temperature: 8o
Flow rate: 1 mL/min
Injection volume: 10 μL
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note—The relative retention times in Table 2 are provided as information that could aid in peak assignment.]
Table 2
| Name | Relative Retention Time |
| 2Z,4Z-Retinoic acida | 0.98 |
| Isotretinoin | 1.0 |
| 2Z,6Z-Retinoic acidb | 1.05 |
| Tretinoin | 1.3 |
a (2Z,4Z,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid.
b (2Z,4E,6Z,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid.
Suitability requirements
Peak-to-valley ratio: NLT 4.0 for 2Z,4Z-retinoic acid and isotretinoin at 355 nm, System suitability solution
Resolution: NLT 1.5 between isotretinoin and 2Z,6Z-retinoic acid at 355 nm, System suitability solution
Relative standard deviation: NMT 5.0% for isotretinoin and tretinoin at 355 nm, Standard solution
Signal-to-noise ratio: NLT 10 at 355 nm and NLT 5 at 310 nm for isotretinoin, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tretinoin in the portion of Isotretinoin taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of tretinoin at 355 nm from the Sample solution
rS = peak response of tretinoin at 355 nm from the Standard solution
CS = concentration of USP Tretinoin RS in the Standard solution (μg/mL)
CU = concentration of Isotretinoin in the Sample solution (μg/mL)
Calculate the percentage of any unspecified impurity, using the 355 nm wavelength, in the portion of Isotretinoin taken:
Result = (rU/rS) × (CS/CU ) × 100
rU = peak response of each impurity at 355 nm from the Sample solution
rS = peak response of isotretinoin at 355 nm from the Standard solution
CS = concentration of USP Isotretinoin RS in the Standard solution (μg/mL)
CU = concentration of Isotretinoin in the Sample solution (μg/mL)
Additionally, calculate the percentage of any unspecified impurity having a relative retention time (RRT) within the range of 0.3–0.5, using the 310 nm wavelength, in the portion of Isotretinoin taken:
Result = (rU/rS) × (CS/CU ) × 100
rU = peak response of each impurity at 310 nm from the Sample solution
rS = peak response of isotretinoin at 310 nm from the Standard solution
CS = concentration of USP Isotretinoin RS in the Standard solution (μg/mL)
CU = concentration of Isotretinoin in the Sample solution (μg/mL)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
| Name | Acceptance Criteria, NMT (%) |
| Tretinoin | 1.0 |
| Any unspecified impuritya | 0.10 |
| Total impuritiesb | 0.5 (USP 1-Dec-2024) |
a For the unspecified impurities having a relative retention time (RRT) within the range of 0.3–0.5, evaluated using both the 355 and 310 nm wavelengths, the higher of the two calculated results is assessed and included in the total.
b Total impurities excludes tretinoin.
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry a sample in a vacuum at room temperature for 16 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Change to read:
Packaging and Storage: Preserve in tight containers, under an atmosphere of an inert gas. Store at room temperature and (USP 1-Dec-2024)
protect from light.
USP Reference Standards 〈11〉
USP Isotretinoin RS
USP Tretinoin RS


