Isopropyl Myristate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
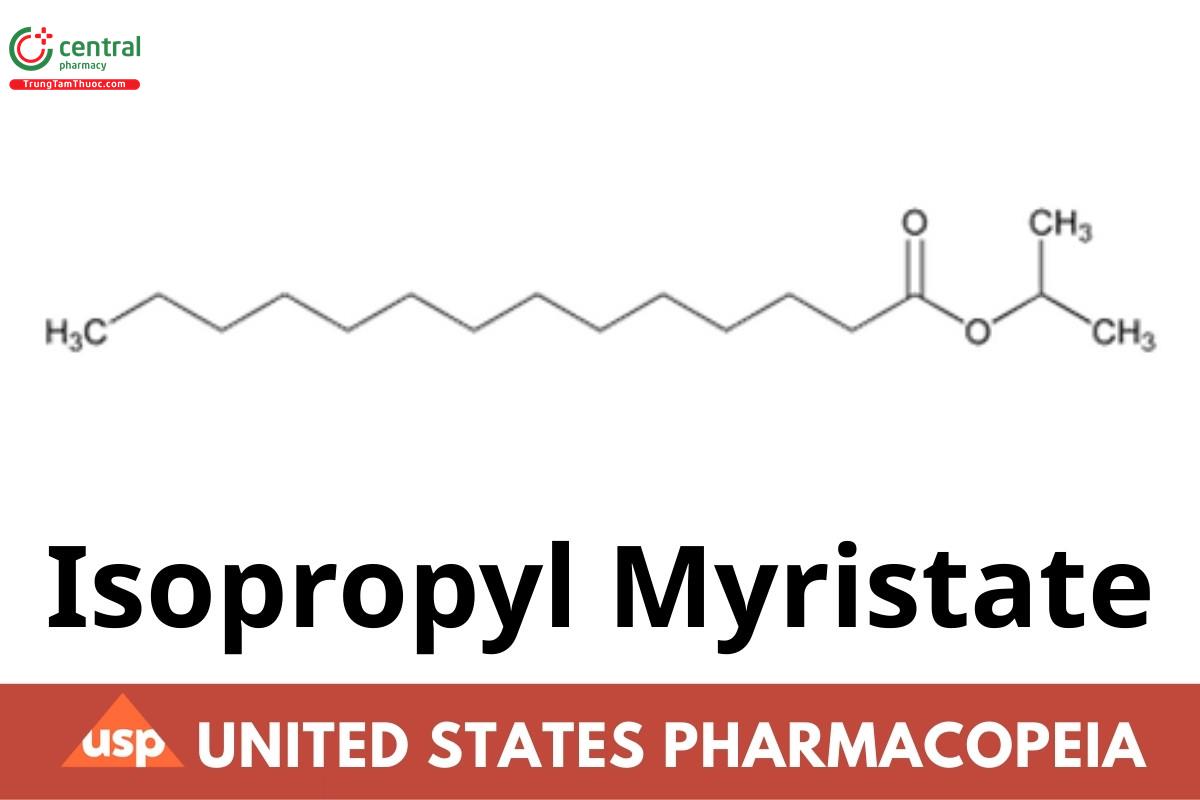
Isopropyl Myristate consists of esters of isopropyl alcohol and saturated high molecular weight fatty acids, principally myristic acid. It contains NLT 90.0% of isopropyl myristate (C17H34O2).
2 IDENTIFICATION
A. The retention times of the major peaks of the Sample solution correspond to those of the System suitability solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
System suitability solution: 5.0 mg/mL of USP Isopropyl Myristate RS and 0.5 mg/mL of USP Isopropyl Palmitate RS in n-hexane Sample solution: 5.0 mg/mL of Isopropyl Myristate in n-hexane
3.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.) Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 15-m fused silica coated with a 1.0-µm layer of stationary phase G27
Temperatures
Injection port: 240°
Detector: 280° Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 150 | — | 150 | 1 |
| 150 | 6 | 230 | 8 |
Carrier gas: Helium Flow rate: 1.5 mL/min Injection volume: 2.0 µL
Injection type: Split injection, split ratio 10:1 Run time: 22 min
3.1.2 System suitability
Sample: System suitability solution
[NOTE—The relative retention times for isopropyl myristate and isopropyl palmitate are 1.0 and 1.3, respectively.] Suitability requirements
Resolution: NLT 6.0 between the isopropyl myristate and isopropyl palmitate peaks
Tailing factor: NMT 2 for the isopropyl myristate peak
Relative standard deviation: NMT 2.0% Analysis
Sample: Sample solution
Calculate the percentage of isopropyl myristate (C17H34O2) in the portion of Isopropyl Myristate taken:
Result = (rU/rT) × 100
rU = peak area of isopropyl myristate
rT = sum of the peak areas of all the peaks, except the solvent peak
Acceptance criteria: NLT 90.0%
4 IMPURITIES
RESIDUE ON IGNITION 〈281〉: NMT 0.1%
5 SPECIFIC TESTS
SPECIFIC GRAVITY 〈841〉: 0.846–0.854
REFRACTIVE INDEX 〈831〉: 1.432–1.436 at 20°
FATS AND FIXED OILS, Acid Value 〈401〉: NMT 1
FATS AND FIXED OILS, Iodine Value 〈401〉: NMT 1
FATS AND FIXED OILS, Saponification Value 〈401〉: 202–212
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
USP REFERENCE STANDARDS 〈11〉
USP Isopropyl Myristate RS USP Isopropyl Palmitate RS


