Isoflurane
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
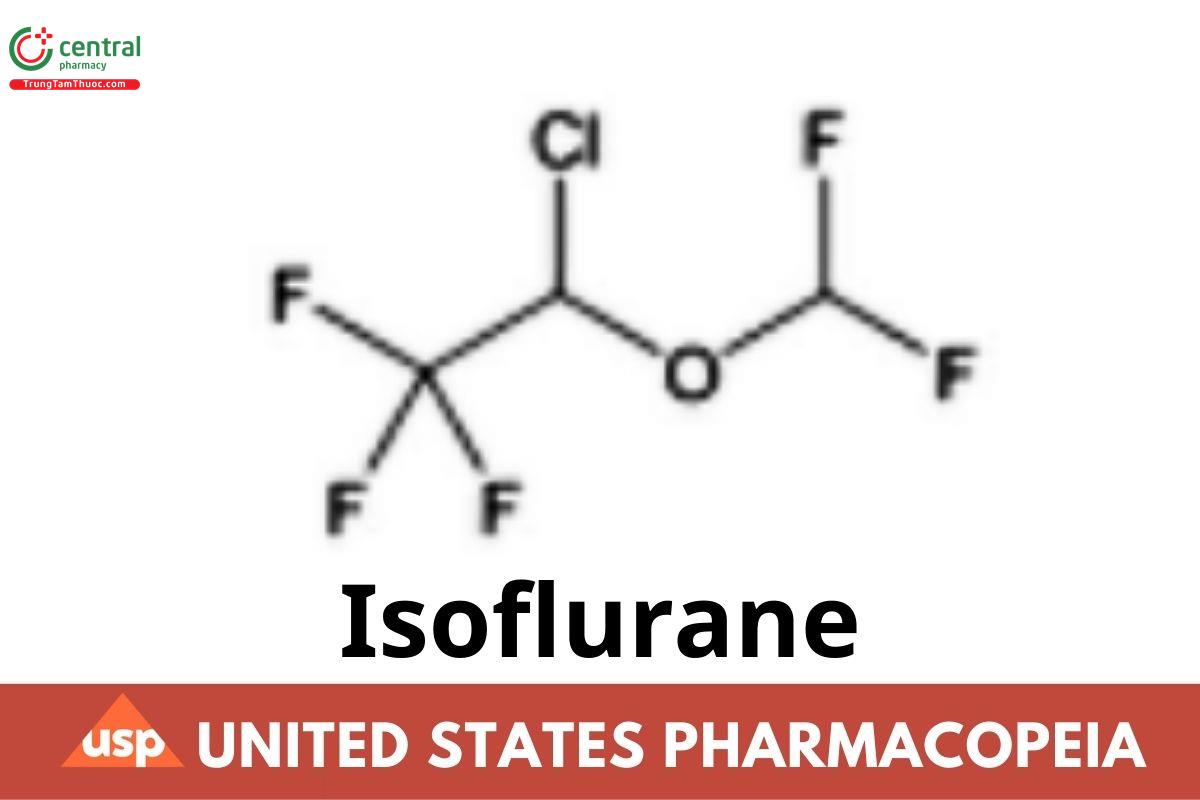
C3H2ClF5O 184.49
Ethane, 2-chloro-2-(difluoromethoxy)-1,1,1-triuoro-;
1-Chloro-2,2,2-triuoroethyl difluoromethyl ether CAS RN®: 26675-46-7; UNII: CYS9AKD70P.
1 DEFINITION
Isoflurane contains NLT 99.9% and NMT 100.0% of isoflurane (C3H2ClF5O).
IDENTIFICATION
The IR absorption spectrum of isoflurane, obtained using a gas cell, exhibits maxima only at the same wavelengths as those of a similar preparation of USP isoflurane RS.
2 ASSAY
Procedure
Analysis: Using the results from the test for Organic Impurities, calculate the percentage of isoflurane (C3H2ClF5O) in the sample of isoflurane taken by subtracting the sum of percentages for the impurities found from 100.0%.
Acceptance criteria: 99.9%–100.0%
3 IMPURITIES
Chloride
Sample solution: Pipet 10 mL of isoflurane into a suitable vessel containing 60 mL of isopropyl alcohol and 4 drops of dilute nitric acid (1:1), and stir to dissolve.
Analysis: Titrate potentiometrically with 0.002 N silver nitrate in isopropyl alcohol VS.
Acceptance criteria: NMT 2.11 mL is required (NMT 10 ppm)
Limit of Fluoride
Use plasticware throughout this test.
Buffer: Dissolve 110 g of sodium chloride and 1 g of sodium citrate in 700 mL of water in a 2-L volumetric flask. Cautiously add 150 g of sodium hydroxide, and dissolve with shaking. Cool to room temperature, and, while stirring, cautiously add 450 mL of glacial acetic acid to the cooled solution. Cool, add 600 mL of isopropyl alcohol, dilute with water to volume, and mix. The pH of this solution is between 5.0 and 5.5. [Note—This solution may be used for 6 weeks if stored at room temperature.]
Solution A: Transfer 55 mg of USP Sodium Fluoride RS to a 25-mL volumetric flask. Add 5 mL of water, and mix to dissolve. Add 1.0 mL of 0.0025 N sodium hydroxide, dilute with water to volume, and mix. Each mL of this solution contains 1 mg of fluoride ion. Store in a tightly closed plastic container. [Note—This solution may be used for 2 weeks if stored in a refrigerator.]
Standard stock solution 1: 2.0 µg/mL of fluoride in water from Solution A
Standard stock solution 2: 6.0 µg/mL of fluoride in water from Solution A
Standard stock solution 3: 10.0 µg/mL of fluoride in water from Solution A
Standard stock solution 4: 20.0 µg/mL of fluoride in water from Solution A
Standard solution 1: 1.0 µg/mL of fluoride in Buffer from Standard stock solution 1
Standard solution 2: 3.0 µg/mL of fluoride in Buffer from Standard stock solution 2
Standard solution 3: 5.0 µg/mL of fluoride in Buffer from Standard stock solution 3
Standard solution 4: 10.0 µg/mL of fluoride in Buffer from Standard stock solution 4
Sample solution: Shake 50.0 mL of isoflurane with 50.0 mL of water for 5 min, and allow the liquids to separate completely. Transfer 25.0 mL of the water layer to a 50-mL volumetric flask, dilute with Buffer to volume, and mix.
Analysis
Samples: Standard solutions 1–4 and Sample solution
(See pH 〈791〉.)
Concomitantly measure the potentials in mV, of Standard solutions 1–4 and the Sample solution with a pH meter capable of a minimum reproducibility of ±0.2 mV and equipped with a fluoride ion electrode and a glass-sleeved calomel reference electrode or a double junction fluoride ion-selective combination electrode. When taking measurements, immerse the electrodes in the solution under test,
which has been transferred to a 150-mL beaker containing a polytef-coated stirring bar. Allow to stir on a magnetic stirrer with an insulated top until equilibrium is attained (1–2 min), and record the potential. Rinse and dry the electrodes between measurements, taking care to avoid damaging the crystal of the fluoride ion electrode.
A satisfactory response is achieved if the difference in potential between the potentials obtained with Standard solution 1 and Standard solution 4, having fluoride concentrations of 1.0 and 10.0 µg/mL, respectively, is in the range of 50–60 mV. Plot the logarithm of the fluoride ion concentrations, in µg/mL, of Standard solutions 1–4 versus potential, in mV. From the measured potential of the Sample solution and the standard response line, determine the concentration, in µg/mL, of fluoride in the Sample solution.
Acceptance criteria: NMT 5 µg/mL of fluoride in the Sample solution [NMT 0.001% (w/v) fluoride in isoflurane]
Nonvolatile Residue
Analysis: Transfer 10.0 mL of isoflurane to a suitable weighed evaporating dish, evaporate with the aid of a current of air or stream of nitrogen to dryness, and dry the residue at 50° for 2 h.
Acceptance criteria: The weight of the residue does not exceed 2.0 mg.
Change to read:
Organic Impurities
Standard stock solution: To a suitable tared vial, fitted with a septum, add 20 mL (29.8 g) of isoflurane. Seal and reweigh the vial to determine the weight of the isoflurane added. To this vial sequentially add 20 µL (30 mg) of USP isoflurane Related Compound A RS, 21 µL (30 mg) of USP isoflurane Related Compound B RS, and 38 µL (30 mg) of USP Acetone RS. Record the weight after the addition of each impurity and determine the total weight.
Calculate the percentage of each impurity in the Standard stock solution:
Result = (WI/WT) × PJ
WI = weight of each impurity added (g)
WT = total weight of the Standard stock solution (g)
PJ = purity of each impurity added (%)
Standard solution: To a suitable tared vial, fitted with a septum, add 10 mL (15 g) of isoflurane. Seal and reweigh the vial to determine the weight of the isoflurane added. To this vial add 300 µL of the Standard stock solution, and record the weight to determine the weight of the Standard stock solution added and the nal weight of the Standard solution.
Calculate the spiked concentration (C ) of each impurity in the Standard solution:
Result = (WI /WT ) × CJ
WI = weight of the Standard stock solution added (g)
WT = total weight of the Standard solution (g)
CJ = concentration of each impurity in the Standard stock solution (%)
System suitability solution: To a suitable vial, fitted with a septum, add 10 mL (15 g) of isoflurane. Seal the vial. To this vial add 100 µL of the Standard stock solution.
Sample solution: isoflurane
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 60-m; fused-silica capillary coated with a 1.0-µm lm of G16
Carrier gas: Helium
Flow rate: 1.7 mL/min (constant ow mode)
Temperatures
Injection port: 175°
Detector: 200°
Column: See Table 1.
Table 1
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
40 | — | 40 | 8 |
40 | 10 | 170 | 4 |
Injection volume: 2 µL
Injection type: Split; split ratio, 23:1
System suitability
Samples: Standard solution and System suitability solution
Suitability requirements
Tailing factor: NMT 1.5 for acetone, Standard solution
Relative standard deviation: NMT 5% each for isoflurane related compound A, isoflurane related compound B, and acetone, Standard solution
Signal-to-noise ratio: NLT 15 for isoflurane related compound B, System suitability solution
Analysis: Injections of isoflurane used to prepare the Standard solution must be made to estimate the amount of known impurities that may be present in the solvent. The nal concentration of each impurity is equal to the concentration of the impurity added plus the concentration inherent in the matrix.
Samples: Standard solution and Sample solution
Calculate the nal concentration (C ) of each impurity in the Standard solution:
Result = [rU/(rS − rU) × CS ] + CS
rU = peak response of each impurity from the isoflurane used as the solvent
rS = peak response of each impurity from the Standard solution
CS = spiked concentration of each impurity in the Standard solution (%)
Calculate the percentage of isoflurane related compound A, isoflurane related compound B, and acetone observed in the Sample solution:
Result = (rU/rS) × CF
rU = peak response of each impurity from the Sample solution
rS = peak response of each impurity from the Standard solution
CF = final concentration of each impurity in the Standard solution
Calculate the percentage of all other impurities:
Result = (rU/rS) × CF × (1/F)
rU = peak response of the impurity from the Sample solution
rS = peak response of isoflurane related compound B from the Standard solution
CF = final concentration of USP isoflurane Related Compound B RS in the Standard solution (%) F = relative response factor relative to isoflurane related compound B (see Table 2)
Acceptance criteria: See Table 2.
Table 2
Name | Relative Retention Timea | Relative Response Factorb | Acceptance Criteria, NMT (%) |
Dichloroisofluranec | 0.41 | 0.28 | 0.003 |
Isoflurane isomerd | 0.43 | 0.87 | 0.003 |
isoflurane related compound A | 0.46 | — | 0.01 |
isoflurane related compound B | 0.56 | 1.00 | 0.007 |
Chloroisofluranee | 0.59 | 0.35 | 0.003 |
Acetone | 0.79 | — | 0.008 |
Isoflurane | 1.00 | — | — |
Any individual unspecified impurity | — | 1.00 | 0.003 |
Total impurities | — | — | 0.1 |
aRelative to isoflurane.
b Relative to isoflurane related compound B.
c 1,1-Dichloro-1-(chlorodifluoromethoxy)-2,2,2-triuoroethane.
d 2-(chlorodifluoromethoxy)-1,1,1-triuoroethane.
e 1,1-Dichloro-1-(difluoromethoxy)-2,2,2-triuoroethane.
4 SPECIFIC TESTS
Refractive Index 〈831〉: 1.2990–1.3005 at 20°
Water Determination 〈921〉, Method I: NMT 0.10%
Acidity or Alkalinity
Sample: Transfer 5 mL of isoflurane and 2 mL of carbon dioxide-free water to a 10-mL glass-stoppered graduated cylinder, shake for 3 min, and allow the layers to separate.
Analysis: Test the aqueous layer (upper) with both red litmus paper and blue litmus paper.
Acceptance criteria: The aqueous layer is neutral to litmus paper. The aqueous phase should not turn the red litmus paper blue, nor should it turn the blue litmus paper red.
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature. Replace the cap securely after each use. USP Reference Standards 〈11〉
USP Acetone RS
USP isoflurane RS
USP isoflurane Related Compound A RS
1-Chloro-2,2,2-triuoroethyl chlorodifluoromethyl ether.
C3HCl2F5O 218.94
USP isoflurane Related Compound B RS
2,2,2-Triuoroethyl difluoromethyl ether.
C3H3F5O 150.05
USP Sodium Fluoride RS


