Ioversol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Change to read:
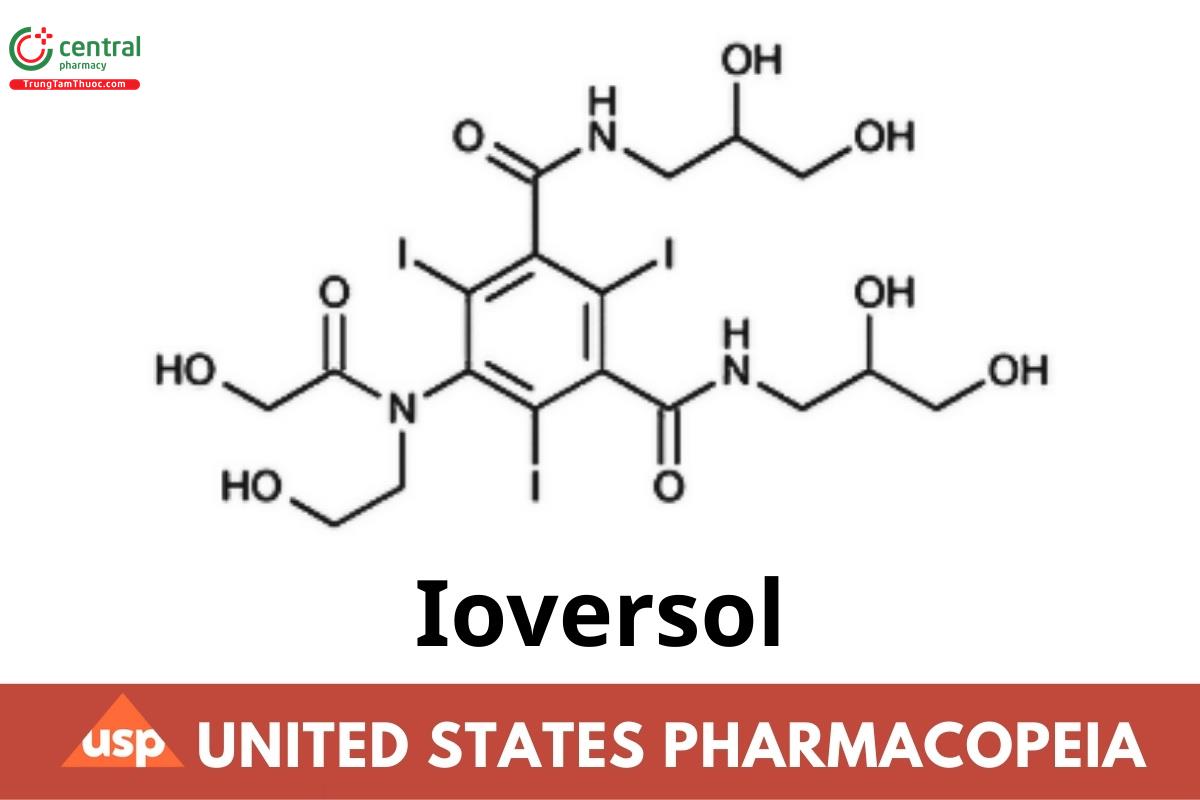
C18H24I3N3O9 807.12 (IRA 1-May-2021)
1,3-Benzenedicarboxamide, N,N'-bis(2,3-dihydroxypropyl)-5-[(hydroxyacetyl) (2-hydroxyethyl)amino]-2,4,6-triiodo-;
N,N'-Bis(2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) glycolamido]-2,4,6-triiodoisophthalamide CAS RN®: 87771-40-2; UNII: N3RIB7X24K.
1 DEFINITION
loversol contains NLT 97.0% and NMT 101.0% of ioversol (C18H24I3N3O9), calculated on the anhydrous basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B.
Sample: About 500 mg
Analysis: Heat the Sample in a crucible.
Acceptance criteria: Violet vapors are evolved.
3 ASSAY
Change to read:
3.1 PROCEDURE
Sample solution:
Transfer about 500 mg of loversol to a glass-stoppered 125-mL conical flask, add 12 mL of 5 N sodium hydroxide, 20 mL of water, and 1 g of powdered zinc. Connect the conical flask to a reflux condenser, and reflux for 30 min. Cool the flask to room temperature, rinse the condenser with 20 mL of water, disconnect the flask from the condenser, and filter the mixture. Rinse the flask and filter thoroughly, adding the rinsings to the filtrate. Add 40 mL of 2 N sulfuric acid, and titrate immediately.
Titrimetric system
Mode: Direct titration
Titrant: 0.05 N silver nitrate VS
Endpoint detection: Potentiometric
Electrode system: Silver-silver chloride double junction reference electrode and silver billet electrode
Analysis
Sample: Sample solution
Titrate with the Titrant determining the endpoint potentiometrically. Each milliliter of 0.05 N silver nitrate is equivalent to 13.45 mg of ioversol (C18H24I3N3O9).
Acceptance criteria: 97.0%-101.0% on the anhydrous basis (IRA 1-May-2021)
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281): NMT 0.1%
Change to read:
4.2 IODINE AND IODIDE
Standard solution: Transfer 2 mL of 0.25 mg/mL of potassium iodide in water to a 50-mL glass-stoppered cylinder, and add 13 mL of water.
Sample solution: Dissolve 2.0 g of loversol in water in a 50-mL glass-stoppered cylinder, and dilute with water to 15 mL..
Analysis: To the 50-mL glass-stoppered cylinders with the Standard solution and Sample solution, add 5 mL each of diluted sulfuric acid and toluene. Shake vigorously, and allow the layers to separate. The toluene layer shows no red color. Add 1 mL of 20 mg/mL of sodium nitrite solution to both the Standard solution and Sample solution, and shake.
Acceptance criteria: Any red color in the toluene layer of the Sample solution is not darker than that of the Standard solution (NMT (IRA 1-May-2021) 0.02% of iodide).
Change to read:
4.3 ORGANIC IMPURITIES
Mobile phase: Acetonitrile and water (0.5: 99.5)
Standard solution: 1.0 µg/mL of USP lohexol Related Compound B RSA (IRA 1-May-2021) and 5.0 µg/mL of USP loversol Related Compound B RS in water
Sample solution: 1000 µg/mL of loversol in water
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm; packing L7
Temperature: 35 ± 0.5°
(IRA 1-May-2021)
Flow rate: 1 mL/min
Injection volume: 50 µL
System suitability
Sample: Standard solution
[NOTE-See Table 1 for relative retention times.]
Suitability requirements
Resolution: NLT 2.0 between iohexol related compound B (IRA 1-May-2021) and ioversol related compound B
Relative standard deviation: NMT 5%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each (IRA 1-May-2021) related compound in the portion of loversol taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each related compound from the Sample solution
rS = average peak response of each corresponding related compound from the Standard solution s
CS = concentration of USP lohexol Related Compound B RS Standard solution (µg/mL) 2A (IRA 1-May-2021) or USP loversol Related Compound B RS in the Standard solution (μg/mL)
CU = concentration of loversol in the Sample solution (µg/mL)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Ioversol | 1.0 | - |
| Iohexol related compound B | 1.8 | 0.10 (IRA 1-May-2021) |
| Ioversol related compound B | 2.1 | 0.50 (IRA 1-May-2021) |
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method 1: NMT 5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. Store at 25°, excursions permitted between 15° and 30°.
Change to read:
USP REFERENCE STANDARDS (11)
USP lohexol Related Compound B RS
5-Amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide.
C14H18I3N3O6 705.03 (IRA 1-May-2021)
USP loversol RS
(IRA 1-May-2021)
USP loversol Related Compound B RS
N,N'-Bis(2,3-dihydroxypropyl)-5-[{(N-(2-hydroxyethyl)amino)-2-oxoethoxy]-2,4,6-triiodoisophthalamide; also known as N,N'-Bis(2,3-dihydroxypropyl)-5-[(N-(2-hydroxyethyl)-carbamoyl)methoxy]-2,4,6-triiodoisophthalamide.
C18H24I3N3O9 807.12 (IRA 1-May-2021)


