Insulin Aspart
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C256H381N65O79S6 5825.54
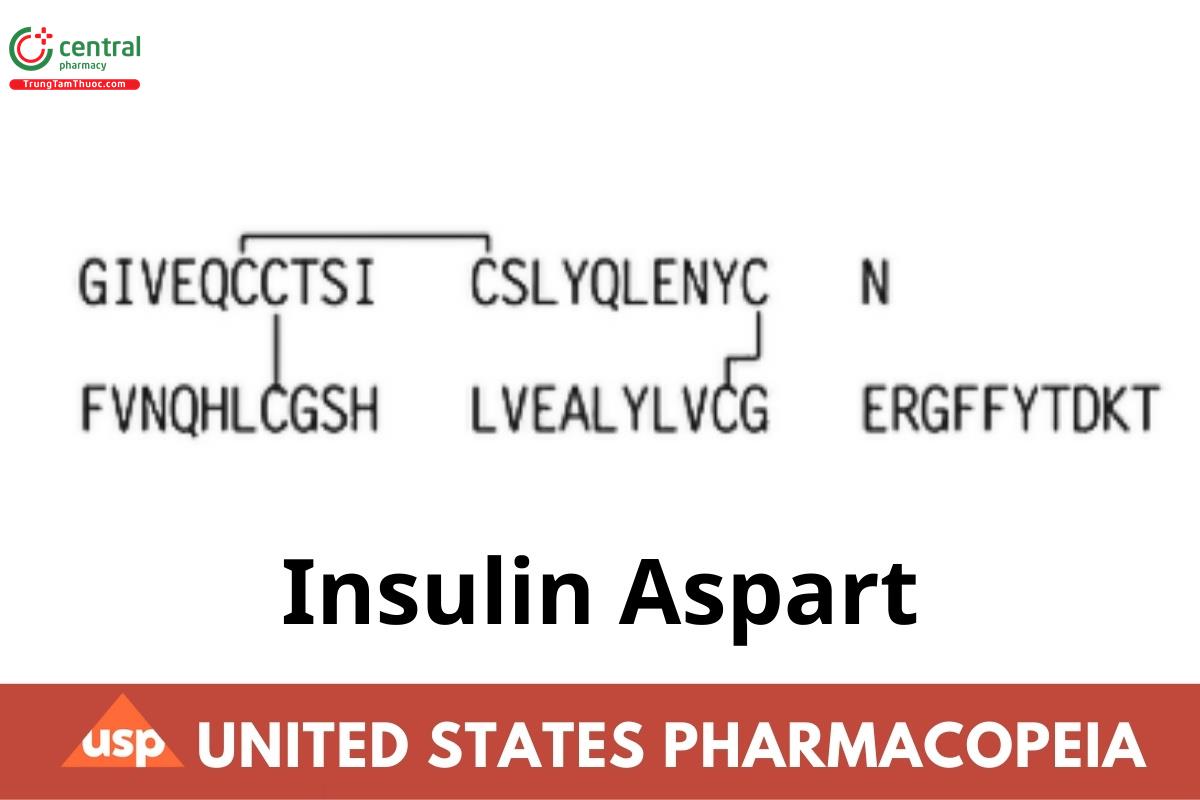
28B-L-Aspartic acid-insulin (human) CAS RN®: 116094-23-6; UNII: D933668QVX.
1 DEFINITION
Insulin Aspart is a two-chain peptide containing 51 amino acids. The A-chain is composed of 21 amino acids, and the B-chain is composed of 30 amino acids. It is identical in primary structure to Insulin Human, except that it has an aspartic acid instead of proline at position 28 of the B-chain. As in human insulin, insulin aspart contains two interchain disulfide bonds, and one intrachain disulfide bond.
Insulin Aspart is produced by methods based on recombinant DNA technology. The presence of host cell DNA in Insulin Aspart is process specific. The capability of the process to clear host-derived DNA requires validation and is determined by validated methods. The host cell-derived protein content is below the limit approved by the competent authority. The content of single-chain precursor is determined by a
suitably sensitive method and is below the limit approved by the competent authority. Insulin Aspart contains NLT 90.0% and NMT 104.0% of
insulin aspart (C256H381 N 6507956) plus A21Asp insulin aspart, B3Asp insulin aspart, B3isoAsp insulin aspart, and B28isoAsp insulin aspart, on
the dried basis. [NOTE-1 USP Insulin Aspart Unit is equivalent to 0.0350 mg of pure insulin aspart.]
2 IDENTIFICATION
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. PHYSICOCHEMICAL ANALYTICAL PROCEDURES FOR INSULINS (121.1), Peptide Mapping
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 60 | 30 | 70 |
| 65 | 0 | 100 |
| 70 | 0 | 100 |
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 10-cm; 3-μm packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 50 μL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 8.0 between the peaks indicated as fragments II and III
Tailing factor: NMT 1.5 for the peaks indicated as fragments II and III
Chromatogram similarity: In the chromatogram from the Standard solution, identify the peaks due to digest fragments I, II, III, and IV. The chromatogram of the Standard solution corresponds to that of the typical chromatogram provided with USP Insulin Aspart RS.
Acceptance criteria: Meets the requirements
3 ASSAY
3.1 PROCEDURE
Solution A: Dissolve 142.0 g of anhydrous sodium sulfate in water, add 13.5 mL of phosphoric acid, and dilute with water to 5 L. Adjust, if necessary, with 10 N sodium hydroxide solution to a pH of 3.6. Mix 9 volumes of this solution with 1 volume of acetonitrile.
Solution B: Acetonitrile and water (1:1)
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 58 | 42 |
| 35 | 58 | 42 |
| 40 | 20 | 80 |
| 45 | 20 | 80 |
| 46 | 58 | 42 |
| 60 | 58 | 42 |
[NOTE-Adjust the Mobile phase composition and the duration of the isocratic elution to obtain a retention time of about 22 min for the insulin aspart and to ensure that B3isoAsp insulin aspart is eluted before the gradient starts.]
System suitability solution: Use an appropriate solution with a content of B3Asp insulin aspart and A21Asp insulin aspart of NLT 1.0%. This may be achieved by storing the Standard solution at room temperature for about 1-3 days.
Standard solution: Dissolve the contents of a vial of USP Insulin Aspart RS in 0.01 N hydrochloric acid to obtain a concentration of 4.0 mg/mL.
Sample solution: Dissolve the sample to be examined in 0.01 N hydrochloric acid to obtain a concentration of 4.0 mg/mL.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.0-mm x 25.0-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for B28isoAsp insulin aspart, insulin aspart, B3Asp insulin aspart plus A21Asp insulin aspart (generally coelute), and B3isoAsp insulin aspart are about 0.9, 1.0, 1.3, and 1.5 min, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the peak for insulin aspart and the peak for A21Asp insulin aspart and B3Asp insulin aspart, System suitability solution
Tailing factor: NMT 1.5 for the insulin aspart peak, System suitability solution
Relative standard deviation: NMT 1.4% for five replicate injections, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of insulin aspart (C256H381N65O79S6) plus B28isoAsp insulin aspart, A21Asp insulin aspart, B3Asp insulin aspart, and B3isoAsp insulin aspart using the areas of the corresponding peaks in the chromatograms from the Sample solution and the Standard solution and the declared content of insulin aspart plus B28isoAsp insulin aspart, A21Asp insulin aspart, B3Asp insulin aspart, and B3isoAsp insulin aspart in the portion of Insulin Aspart taken:
Result = (rIA(U) + rB28iso(U) + rA21(U) + rB3(U) + rB3iso(U))/(rIA(S) + rB28iso(S) + rA21(S) + rB3(S) + rB3iso(S) × (CS/CU) × 100
rIA(U) = peak response of insulin aspart from the Sample solution
rB28iso(U) = peak response of B28isoAsp insulin aspart from the Sample solution
rA21(U) = peak response of A21Asp insulin aspart from the Sample solution
rB3(U) = peak response of B3Asp insulin aspart from the Sample solution
rB3iso(U) = peak response of B3isoAsp insulin aspart from the Sample solution
rIA(S) = peak response of insulin aspart from the Standard solution
rB28iso(S) = peak response of B28isoAsp insulin aspart from the Standard solution
rA21(S) = peak response of A21 Asp insulin aspart from the Standard solution
rB3(S) = peak response of B3Asp insulin aspart from the Standard solution
rB3iso(S) = peak response of B3isoAsp insulin aspart from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 90.0%-104.0% on the dried basis
4 PRODUCT-RELATED SUBSTANCES AND IMPURITIES
RESIDUE ON IGNITION (281): NMT 6.0% determined on 0.2 g (dried substance)
PRODUCT-RELATED SUBSTANCES
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed
as directed in the Assay using the normalization procedure.
Acceptance criteria
Individual impurities: NMT 1.0% of B28isoAsp insulin aspart; NMT 2.0% total of the peaks due to A21Asp insulin aspart, B3Asp insulin aspart, and B3isoAsp insulin aspart
Total of other impurities: NMT 1.5%
PHYSICOCHEMICAL ANALYTICAL PROCEDURES FOR INSULINS (121.1), Limit of High Molecular Weight Proteins: Meets the requirements; NMT 0.5%
5 SPECIFIC TESTS
INSULIN ASSAYS (121), Assay, Bioidentity Test: Meets the requirements
BACTERIAL ENDOTOXINS TEST (85): It contains NMT 10 USP Endotoxin Units/mg of Insulin Aspart.
MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62): The total aerobic count does not exceed 300 cfu/g, the test being performed on a portion of about 0.2 g.
LOSS ON DRYING (731)
Sample: About 200 mg
Analysis: Dry the Sample at 105° for 24 h.
Acceptance criteria: NMT 10.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers. Store in a freezer and protect from light.
LABELING: Label it to indicate that it has been produced by methods based on recombinant DNA technology.
USP REFERENCE STANDARDS (11)
USP Insulin Aspart RS


