Insulin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
To view the Notice from the Expert Committee that posted in conjunction with this accelerated revision, please click
https://www.uspnf.com/rb-insulin-20190401
Change to read:
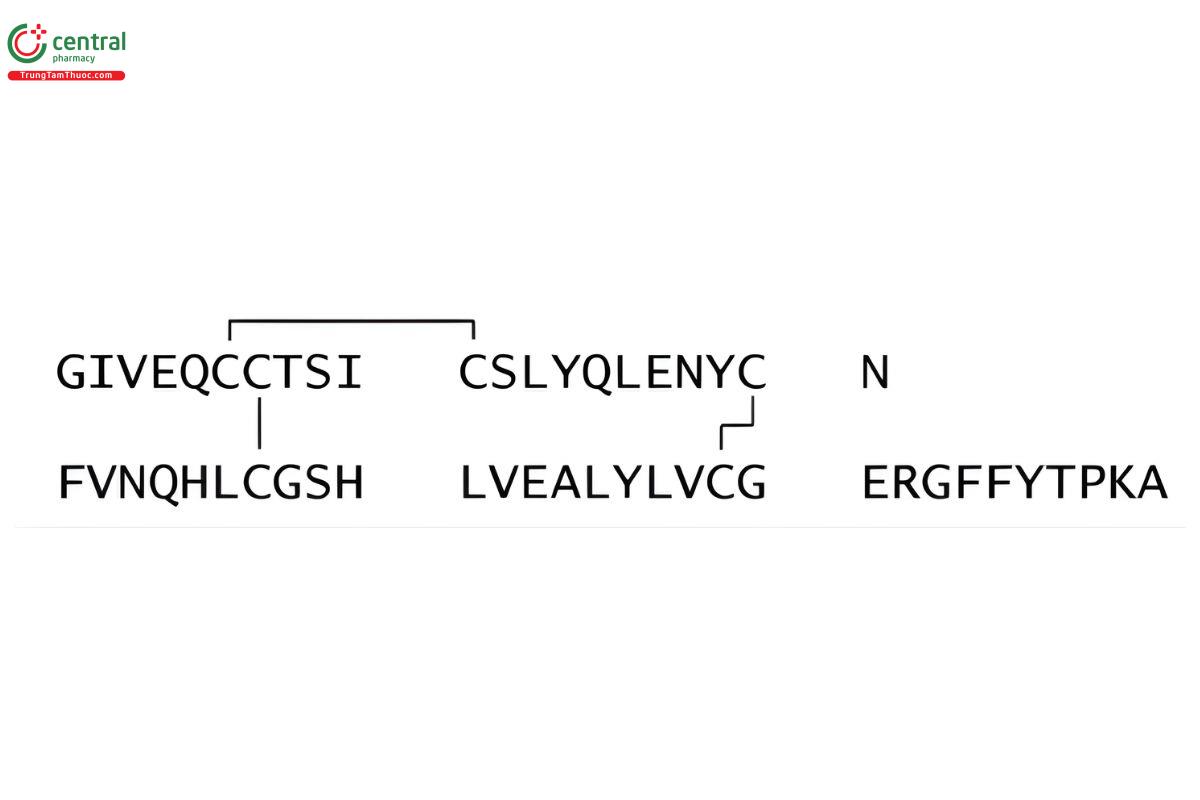
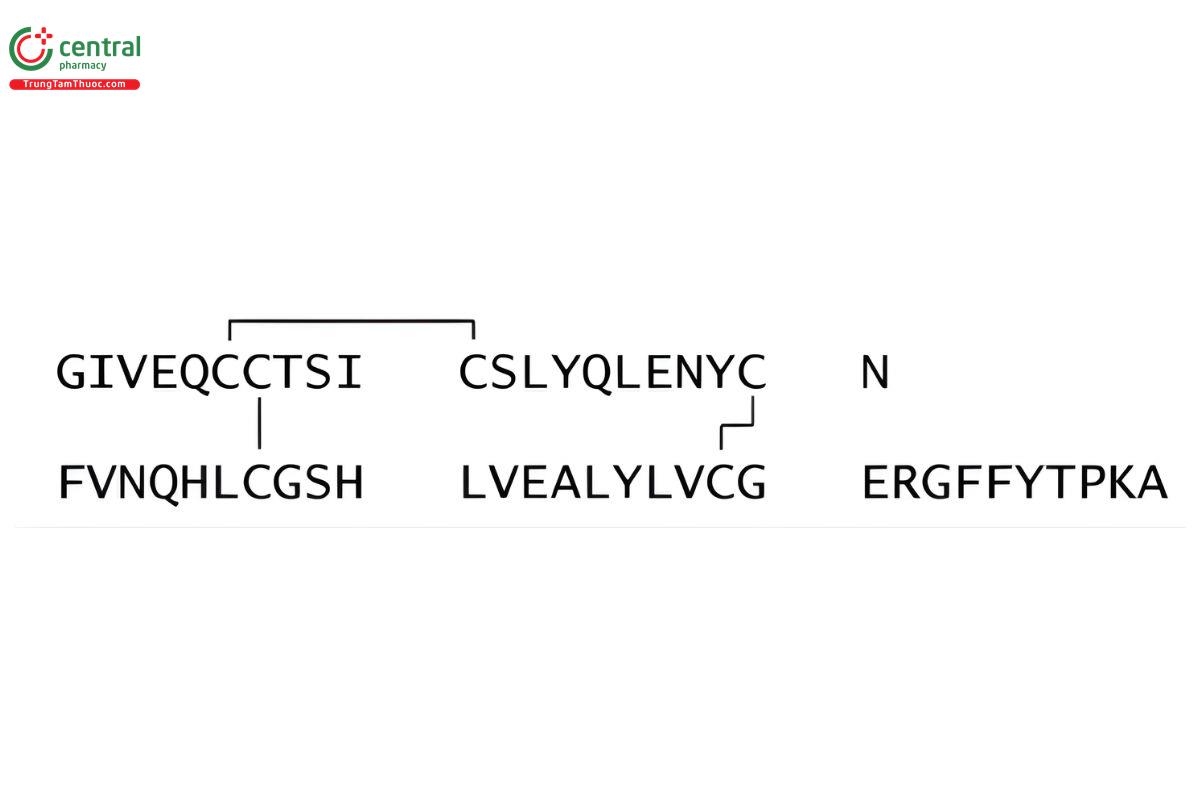
Insulin (pig) CAS RN®: 12584-58-6.
(RB 1-May-2019)
1 DEFINITION
Change to read:
Insulin is a two-chain peptide hormone consisting of 51 amino acids, and its structure corresponds to native insulin produced in vivo by the beta cells of the pancreas. The A-chain is composed of 21 amino acids, and the B-chain is composed of 30 amino acids. (USP 1-May-2019) It is obtained from the pancreas of healthy (RB 1-May-2019) porcine animals, (RB 1-May-2019) used for food by humans. Its potency is NLT 26.5 USP Insulin Units/mg, calculated on the dried basis; Insulin labeled as puriffied contains NLT 27.0 USP Insulin Units/mg, calculated on the dried basis. (USP 1-May-2019)
[Note—1 USP Insulin Unit is equivalent to (RB 1-May-2019) 0.0345 mg of pure Insulin derived from pork.]
2 IDENTIFICATION
Change to read:
A. The retention time of the major peak in the Sample solution corresponds to that
(RB 1-May-2019) of the Identification solution, as obtained in the Assayand no other signi cant peaks are observed. (RB 1-May-2019)
[Note—It may be necessary to inject a mixture of Sample solution and Identification solution.]
Delete the following:
B. Peptide Mapping
Sulfate buffer: 2.0 M ammonium sulfate and 0.5 M sulfuric acid (1:1)
Enzyme solution: 500 units/mL of Staphylococcus aureus V-8 protease activity in water
HEPES buffer: 0.1 M HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). Adjust with 5 M sodium hydroxide to a pH of 7.5 before diluting with water to a final volume.
Solution A: Acetonitrile, water, and Sulfate buffer (100:700:200)
Solution B: Acetonitrile, water, and Sulfate buffer (400:400:200)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 60 | 30 | 70 |
| 65 | 0 | 100 |
| 70 | 0 | 100 |
| 71 | 90 | 10 |
| 86 | 90 | 10 |
Standard digest solution: 2 mg/mL of USP Insulin RS of the appropriate species in 0.01 N hydrochloric acid. Transfer 500 μL of the resulting solution to a clean vial. Add 2.0 mL of HEPES buffer and 400 μL of Enzyme solution, and incubate at 25° for 6 h. Quench the digestion by adding 2.9 mL of Sulfate buffer.
Sample digest solution: 2 mg/mL of Insulin in 0.01 N hydrochloric acid, mix to dissolve. Transfer 500 μL of the resulting solution to a clean vial. Add 2.0 mL of HEPES buffer and 400 μL of Enzyme solution, and incubate at 25° for 6 h. Quench the digestion by adding 2.9 mL of
Sulfate buffer.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 10-cm; packing L1
Column temperature: 40°
Flow rate: 1 mL/min
System suitability
Sample: Standard digest solution
Suitability requirements
Chromatogram comparability: The chromatogram of the Standard digest solution corresponds to that of the reference chromatogram provided with USP Insulin RS of the appropriate species.
Resolution: NLT 1.9 between digest fragments II and III.
[Note—Fragment I elutes at the same time in insulin derived from pork and Insulin Human; Fragment II elutes at the same time in Insulin
Human and insulin derived from beef and pork; and Fragment III elutes at the same time in insulin derived from beef and pork.]
Tailing factor: NMT 1.5
Analysis
Samples: Standard digest solution and Sample digest solution
Using the gradient program, run a blank. Separately inject equal volumes of the Standard digest solution and the Sample digest solution, and record the responses of each peak.
Acceptance criteria: The chromatographic profile of the Sample digest solution corresponds to that of the Standard digest solution. (USP 1- May-2019)
Add the following:
B. Physicochemical Analytical Procedures for Insulins 〈121.1〉, Peptide Mapping: Proceed as directed in the chapter, except for the Mobile phase and System suitability.
Mobile phase: See Table 1.
Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 60 | 30 | 70 |
| 65 | 0 | 100 |
| 70 | 0 | 100 |
| 71 | 90 | 10 |
| 86 | 90 | 10 |
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 1.9 between digest fragments II and III
[Note—Fragment I elutes at the same time in insulin derived from pork and Insulin Human; Fragment II elutes at the same time in Insulin
Human and insulin derived from beef and pork; and Fragment III elutes at the same time in insulin derived from beef and pork.]
Tailing factor: NMT 1.5 for digest fragments II and III
Chromatogram similarity: The chromatogram of the Standard solution corresponds to that of the reference chromatogram provided with
USP Insulin Pork RS.
Acceptance criteria: Meets the requirements (USP 1-May-2019)
Add the following:
C. Insulin Assays 〈121〉, Assay, Bioidentity Test: Meets the requirements (USP 1-May-2019)
3 ASSAY
Change to read:
Procedure
Solution A: Dissolve 28.4 g of anhydrous sodium sulfate in 1000 mL of water. Pipet 2.7 mL of phosphoric acid into the solution, and adjust with ethanolamine to a pH of 2.3, if necessary.
Mobile phase: Acetonitrile and Solution A (26:74). [Note—The acetonitrile is warmed to a temperature of NLT 20° to avoid precipitation.]
System suitability solution: 1.5 mg/mL of Insulin in 0.01 N hydrochloric acid. Allow to stand at room temperature for NLT 3 days to obtain a solution containing NLT 5% of A-21 desamido insulin.
[Note—The Identification solution, Standard solution, and Sample solution may be stored at room temperature for up to 12 h or in a refrigerator for up to 48 h.]
Identification solution: 0.6 mg/mL of USP Insulin Pork RS (RB 1-May-2019) in 0.01 N hydrochloric acid
Standard solution: 1.5 mg/mL of USP Insulin Pork RS (RB 1-May-2019) in 0.01 N hydrochloric acid
(RB 1-May-2019)
Sample solution: 1.5 mg/mL of Insulin in 0.01 N hydrochloric acid
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 15-cm; packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 20 μL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 2.0 between insulin and A-21 desamido insulin, System suitability solution
Tailing factor: NMT 1.8 for the insulin peak, System suitability solution
Relative standard deviation: NMT 1.6%, Standard solution
Analysis
Samples: Identification solution, Standard solution, and Sample solution
Measure the peak responses for insulin and A-21 desamido insulin, using the chromatogram of the Identification solution to identify the insulin peaks.
(RB 1-May-2019) Calculate the potency on the undried basis, in USP Insulin Units/mg, of Insulin in the Sample solution:
Result = (ΣrU/ΣrS) × (CS/CU)
rU = sum of the peak responses of insulin and A-21 desamido insulin from the Sample solution
rS = sum of the peak responses of insulin and A-21 desamido insulin from the Standard solution
CS = concentration of (RB 1-May-2019) USP Insulin Pork RS in the Standard solution (USP Insulin Units/mL)
CU = concentration of Insulin in the Sample solution (mg/mL) (RB 1-May-2019)
Acceptance criteria: NLT 26.5 USP Insulin Units/mg on the dried basis; Insulin labeled as puriffied contains NLT 27.0 USP Insulin Units/mg on the dried basis.
OTHER COMPONENTS
Change to read:
Zinc Determination 〈591〉
Acceptance criteria: NMT 1.0% on the dried basis (USP 1-May-2019)
PRODUCT-RELATED SUBSTANCES AND IMPURITIES
Change to read:
Product-Related Substances (USP 1-May-2019)
Solution A: Dissolve 28.4 g of anhydrous sodium sulfate in 1000 mL of water. Pipet 2.7 mL of phosphoric acid into the solution, and adjust with ethanolamine to a pH of 2.3, if necessary.
Solution B: Acetonitrile and Solution A (18:82)
Solution C: Acetonitrile and Solution A (50:50)
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution B(%) | Solution C (%) |
| 0 | 100 | 0 |
| 2.5 | 100 | 0 |
| 0 | 81 | 19 |
| 60 | 81 | 19 |
| 85 | 36 | 64 |
| 91 | 36 | 64 |
| 92 | 81 | 19 |
System suitability solution: 1.5 mg/mL of Insulin in 0.01 N hydrochloric acid. Allow to stand at room temperature for NLT 3 days to obtain a solution containing NLT 5% of A-21 desamido insulin.
[Note—Standard solutions A–C may be stored at room temperature for up to 12 h and in a refrigerator for up to 48 h.]
Standard solution A: 3.75 mg/mL of USP Insulin Pork RS (RB 1-May-2019) in 0.01 N hydrochloric acid
(RB 1-May-2019)
Standard solution B: 0.375 mg/mL of
(RB 1-May-2019) USP Insulin Pork RS in 0.01 N hydrochloric acid prepared as follows. Pipet 1 mL of
Standard solution A into a 10-mL volumetric flask, dilute with 0.01 N hydrochloric acid to volume, and mix.
Standard solution C: 0.0375 mg/mL of
(RB 1-May-2019) USP Insulin Pork RS in 0.01 N hydrochloric acid prepared as follows. Pipet 1 mL of
Standard solution B into a 10-mL volumetric flask, dilute with 0.01 N hydrochloric acid to volume, and mix.
Sample solution: 3.75 mg/mL of Insulin in 0.01 N hydrochloric acid. Prepare the solution in a capped vial, cap the vial, and shake gently to dissolve. Store the solution for NMT 2 h at room temperature or for NMT 12 h in a refrigerator.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 25-cm; packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 20 μL
System suitability
Samples: System suitability solution, Standard solution A, Standard solution B, and Standard solution C
[Note—Adjust the Mobile phase composition and the duration of the isocratic elution to obtain a retention time of about 31 min for insulin,
with the A-21 desamido insulin eluting just prior to the start of the gradient elution phase.]
Suitability requirements for the System suitability solution
Resolution: NLT 2.0 between insulin and A-21 desamido insulin
Tailing factor: NMT 1.8 for the insulin peak
Suitability requirements for the Standard solutions
Calculate the factor X :
X1 = (rB/rA) × D
rB = peak response from Standard solution B
rA = peak response from Standard solution A
D = dilution factor, 10
Result: Between 0.91 and 1.09
Calculate the factor X :
X2 = (rC/rA) × D
rC = peak response from Standard solution C
rA = peak response from Standard solution A
D = dilution factor, 100
Result: Between 0.7 and 1.3
Analysis
Sample: Sample solution
Calculate the percentage of insulin, A-21 desamido insulin, and other insulin-related substances (USP 1-May-2019) in the portion of
Insulin taken:
Calculate the percentage of Insulin (%I):
Result = (rl/rT) × 100
rl = peak response of insulin from the Sample solution
rT = sum of the responses of all the peaks from the Sample solution
Calculate the percentage of A-21 desamido insulin (%D):
Result = (rD/rT) × 100
rD = peak response of A-21 desamido insulin from the Sample solution
rT = sum of the responses of all the peaks from the Sample solution
Calculate the percentage of other insulin-related substances: (USP 1-May-2019)
Result = 100 − (%I + %D)
Acceptance criteria: NMT 10.0% of A-21 desamido insulin, and NMT 5.0% of other insulin-related substances (USP 1-May-2019)
(RB 1-May-2019)
Change to read:
Physicochemical Analytical Procedures for Insulins 〈121.1〉, Limit of High Molecular Weight Proteins: Meets the requirements (USP 1-May-2019)
Acceptance criteria: NMT 1.0%
PROCESS-RELATED IMPURITIES
Add the following:
Proinsulin Content: NMT 10 ng/mg, determined by a validated method (USP 1-May-2019)
4 SPECIFIC TESTS
Delete the following:
Insulin Assays 〈121〉, Assay, Bioidentity Test: Meets the requirements (USP 1-May-2019)
Loss on Drying 〈731〉
Sample: 200 mg
Analysis: Dry the Sample at 105° for 16 h.
Acceptance criteria: NMT 10.0%
Delete the following:
Zinc Determination 〈591〉, Procedure, Dithizone Method
Sample: 10 mg
Acceptance criteria: NMT 1.0% on the dried basis (USP 1-May-2019)
Bacterial Endotoxins Test 〈85〉: NMT 10 USP Endotoxin Units/mg of insulin
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total bacterial count does not exceed 3 × 102 cfu/g, the test being performed on a portion of about 0.2 g, accurately weighed.
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store, protected from light, in a freezer.
Change to read:
Labeling: Label it
(RB 1-May-2019) as pork
(RB 1-May-2019) . If the Insulin is puriffied, label it as such.
Change to read:
USP Reference Standards 〈11〉
(RB 1-May-2019)
USP Insulin Pork RS


