Indocyanine Green
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Change to read:
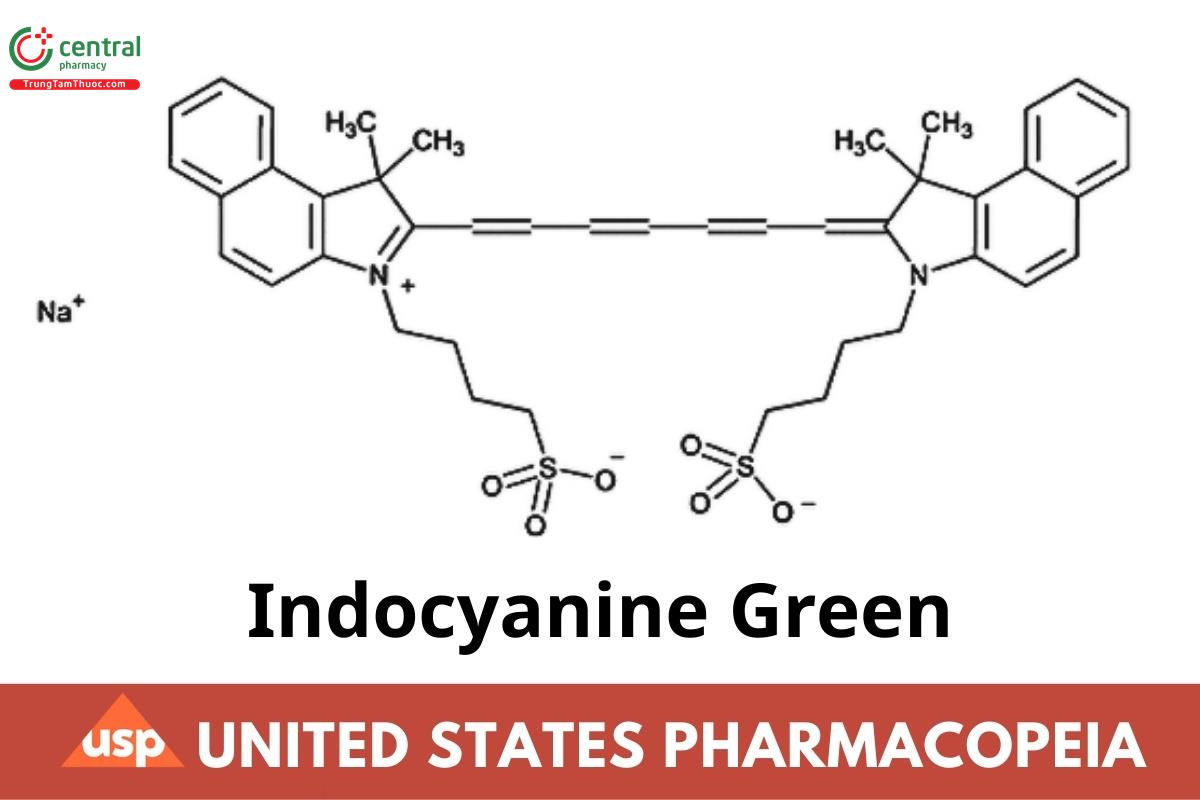
C43H47N2NaO6S2 774.97 (USP 1-Aug-2022)
1H-Benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e]indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-, hydroxide, inner salt, sodium salt;
2-[7-[1,1-Dimethyl-3-(4-sulfobutyl)benz[e]indolin-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-1H-benz[e]indolium hydroxide, inner salt, sodium salt CAS RN®: 3599-32-4; UNII: IX6J1063HV.
Change to read:
1 DEFINITION
Indocyanine Green contains NLT 89.0% and NMT 100.0% of indocyanine green (C43H47N2NaO6S2), calculated on the dried basis. It contains
NMT 5.0% of sodium iodide (Nal), (USP 1-Aug-2022) calculated on the dried basis.
2 IDENTIFICATION
2.1 A. IDENTIFICATION TESTS GENERAL (191), Chemical Identification Tests, Sodium and Sulfate
Analysis: Incinerate a portion of Indocyanine Green and use the residue.
Acceptance criteria: Meets the requirements
Change to read:
2.2 B.
Sample solution: 0.05 mg/mL of Indocyanine Green in water
Analysis: Add 10 drops of sodium hydroxide TSA (USP 1-Aug-2022) to the Sample solution and heat to about 60°. Add 10 drops of Hydrogen peroxide TS and mix.
Acceptance criteria: A red color develops within 4 min, which on standing, fades to a pale orange.
3 ASSAY
Change to read:
3.1 PROCEDURE
Standard solution: 2 µg/mL of USP Indocyanine Green RS in methanol
Sample solution: 2 µg/mL of Indocyanine Green in methanol
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).) (USP 1-Aug-2022)
Mode: UV-Vis
Analytical wavelength: 785 nm
Cell: 1 cm
Blank: Methanol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of indocyanine green (C43H47N2NaO6S2) in the portion of Indocyanine Green taken:
Result = (AU/AS) × (CS/CU) × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of USP Indocyanine Green RS in the Standard solution (µg/mL)
CU = concentration of Indocyanine Green in the Sample solution (µg/mL)
Acceptance criteria: 89.0%-100.0% on the dried basis
4 OTHER COMPONENTS
Change to read:
4.1 SODIUM IODIDE
Sample: 200 mg of Indocyanine Green
Titrimetric system
(See Titrimetry (541).) (USP 1-Aug-2022)
Mode: Direct titration
Titrant: 0.01 N silver nitrate VS
Electrode system: Silver-glass
Endpoint detection: Potentiometric
Analysis: Dissolve the Sample in 100 mL of water. Add 1 mL of nitric acid and mix. Titrate with Titrant to a potentiometric endpoint.
Calculate the percentage of sodium iodide (Nal) in the portion of Indocyanine Green taken:
Result = [(V x N x F)/W] x 100
V = volume of Titrant consumed by the Sample (mL)
N = Titrant concentration, in normality (mEq/mL)
F = equivalency factor of sodium iodide, 149.9 mg/mEq
W = weight of Sample taken (mg)
Acceptance criteria: NMT 5.0% on the dried basis
Delete the following:
5 IMPURITIES
ARSENIC (211), Procedures, Method II: NMT 8 ppm
LEAD (251)
Sample solution: A 5-mL portion of the solution prepared for the test for Arsenic (211), Procedures, Method II
Analysis: Proceed as directed for the limit test of Lead (251). Use 3 mL of Ammonium Citrate Solution, 1 mL of Potassium Cyanide Solution, and 0.5 mL of Hydroxylamine Hydrochloride Solution.
Acceptance criteria: NMT 10 ppm (USP 1-Aug-2022)
6 SPECIFIC TESTS
STERILITY TESTS (71): Where the label states that Indocyanine Green is sterile, it meets the requirements.
Change to read:
BACTERIAL ENDOTOXINS TEST (85): Where the label states that Indocyanine Green is sterile or must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Indocyanine Green is used can be met. (USP 1-Aug-2022)
LOSS ON DRYING (731)
Analysis: Dry under vacuum at 50° for 3 h.
Acceptance criteria: NMT 6.0%
7 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. Store at 25°, excursions permitted between 15° and 30°.
Change to read:
LABELING: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins. (USP 1-Aug-2022)
USP REFERENCE STANDARDS (11)
USP Indocyanine Green RS


