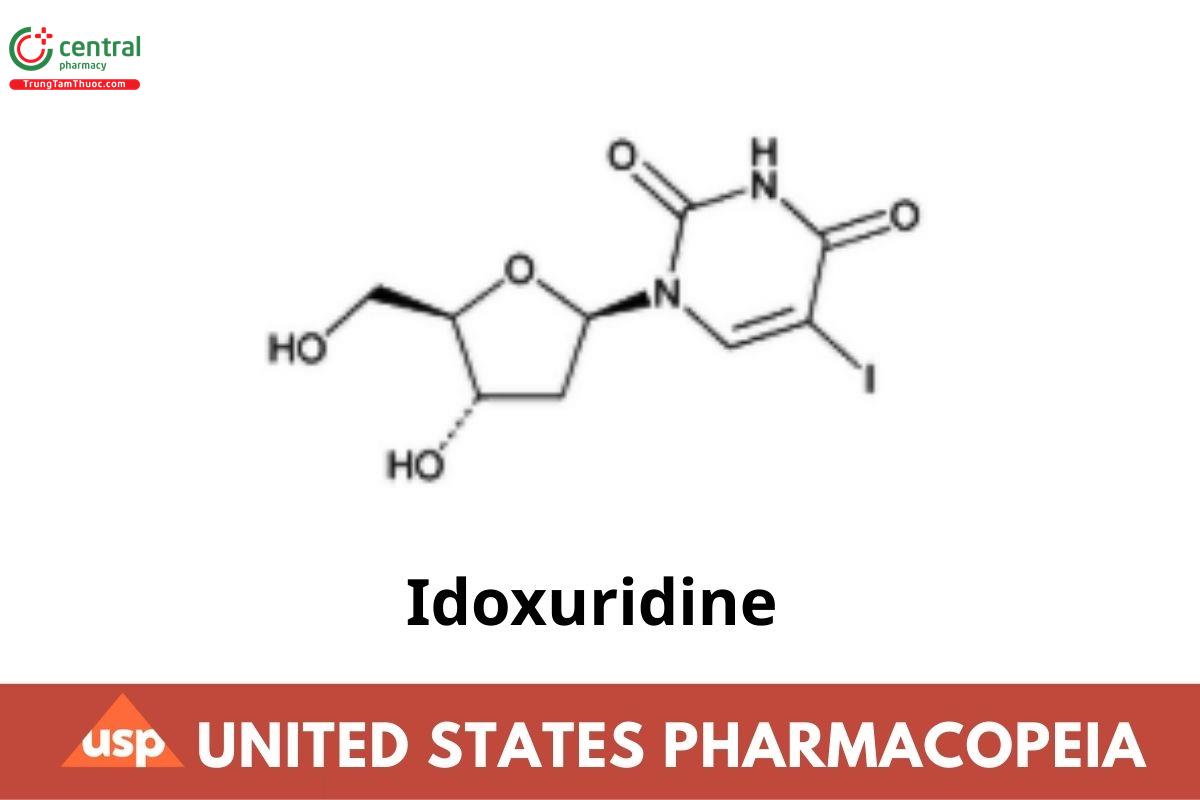
Idoxuridine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Idoxuridine contains NLT 98.0% and NMT 101.0% of idoxuridine (C₉H₁₁IN₂O₅), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. ▲Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M▲ (CN 1-May-2020)
Change to read:
B. ▲Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U▲ (CN 1-May-2020)
Analytical wavelength: 279 nm
Buffer: pH 12.0 solution prepared as follows. Dissolve 7.46 g of potassium chloride and 24 mL of 1 N sodium hydroxide in 2000 mL of water.
Sample solution: 35 µg/mL in Buffer
Acceptance criteria: Absorptivities, calculated on the dried basis for the Sample solution only, do not differ by more than 2.0%.
3 ASSAY
Procedure
Sample: 250 mg
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N sodium methoxide in toluene VS
Endpoint detection: Visual
Analysis:
Dissolve the Sample in 20 mL of dimethylformamide that has previously been neutralized with Titrant, and add 3 mg/mL of thymol blue in methanol as the indicator. Titrate with Titrant to a blue endpoint. Take precautions against absorption of atmospheric carbon dioxide. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium methoxide is equivalent to 35.41 mg of idoxuridine (C₉H₁₁IN₂O₅).
Acceptance criteria: 98.0%–101.0% on the dried basis
4 SPECIFIC TESTS
Loss on Drying 〈731〉
Sample: 500 mg
Analysis: Dry the Sample under vacuum at 60° for 2 h.
Acceptance criteria: NMT 1.0%
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
USP Reference Standards 〈11〉
USP Idoxuridine RS


