Hydroxypropyl Pea Starch
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
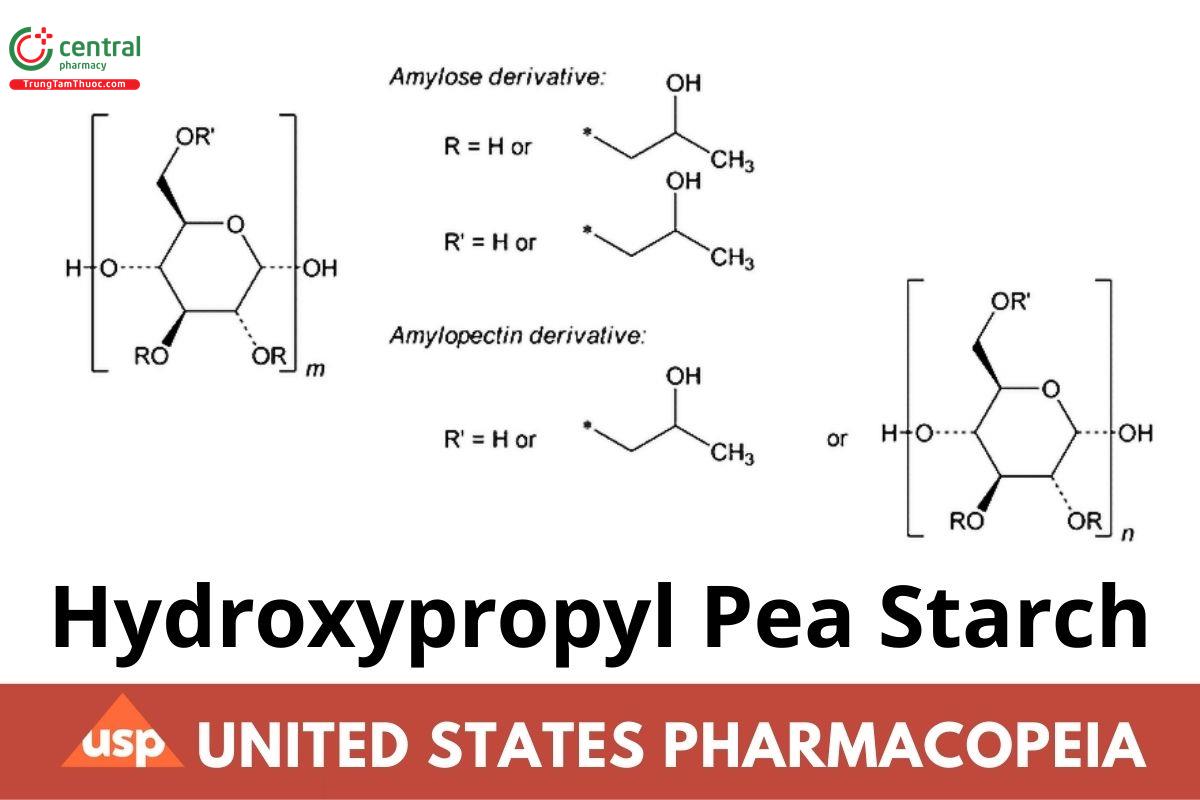
For the Amylose derivative, m is about 300-1000.
1 DEFINITION
Hydroxypropyl Pea Starch is partially substituted 2-hydroxy propylether obtained from pea starch by a chemical modification of etherification with propylene oxide. In addition, this starch may be partially hydrolyzed using acids or enzymes to obtain thinned starch. It contains NLT 2.0% and NMT 7.0% of hydroxypropyl groups, on the dried basis.
2 IDENTIFICATION
2.1 A. PROCEDURE
Analysis: Examine under a microscope, using NLT 20x magnification and a mixture of Glycerin and water (1:1) as a mounting agent.
Acceptance criteria: It presents a majority of large elliptical granules 25-45 µm in size, sometimes irregular or reniform. It also presents a minority of small rounded granules 5-8 µm in size. Granules can present cracks or irregularities. Sometimes, granules show barely visible concentric striations. Exceptionally, granules show a slit along the main axis. Between orthogonally oriented polarizing plates or prisms, the granules show a distinct black cross.
2.2 B. PROCEDURE
Sample solution: Suspend 1 g of Hydroxypropyl Pea Starch in 50 mL of water, boil for 1 min, and cool.
Acceptance criteria: A translucent or clear mucilage is formed.
2.3 C. PROCEDURE
Analysis: To 1 mL of the Sample solution obtained in Identification test B add 0.05 mL of iodine and potassium iodide TS 2.
Acceptance criteria: An orange-red to dark blue color is produced, which disappears upon heating.
2.4 D. PROCEDURE
Ninhydrin solution: Dissolve 3 g of ninhydrin in 100 mL of a 45.5-g/L solution of sodium metabisulfite.
Diluted sulfuric acid: 98 g/L of H2SO4
Sample: 100 mg of Hydroxypropyl Pea Starch
Analysis: Transfer the Sample to a 100-mL volumetric flask, and add 12.5 mL of Diluted sulfuric acid. Place the flask in a water bath, and heat until the Sample is dissolved. Cool, and dilute with water to 100 mL.. [CAUTION-When sulfuric acid is miscible with water, it produces intense heat.]
Pipet 1 mL of this solution to a glass-stoppered, 25-mL graduated test tube and, with the tube immersed in cold water, add drop-wise 8 mL of sulfuric acid. Mix well, and place the tube in a boiling water bath for exactly 3 min. Immediately transfer the tube to an ice bath until the solution is chilled. Add 0.6 mL of Ninhydrin solution, carefully allowing the reagent to run down the walls of the test tube. Immediately shake the tube well, and place it in a water bath at 25° for 100 min. Dilute with sulfuric acid to 25 mL [CAUTION-Use sulfuric acid cautiously.], and mix by inverting the tube several times. Do not shake.
Acceptance criteria: A violet color develops within 5 min due to the presence of hydroxypropyl groups (starch ether).
3 ASSAY
3.1 PROCEDURE FOR HYDROXYPROPYL GROUPS
Deuterium chloride solution: Dilute 1 mL of deuterium chloride (38% w/w) with 5 mL of deuterium oxide.
Internal standard solution: Dissolve 50.0 mg of sodium 3-trimethylsilyl-1-propane sulfonate in about 5 g of deuterium oxide, weighed to the nearest 0.1 mg. Store in a sealed bottle.
Sample solution: Disperse 20 g of Hydroxypropyl Pea Starch in 200.0 mL of carbon dioxide-free water at room temperature. Agitate for 15
min, and filter. Repeat the operation two more times. If poor dispersibility or slow filtration is observed, use refrigerated carbon dioxide-free water for the washing operation. Dry the washed starch for NLT 4 h in vacuum at 30 ± 5o. Weigh 12.0 mg of this sample in a 5-mm NMR tube. Add 0.75 mL of deuterium oxide and 0.1 mL of Deuterium chloride solution. Cap the tube, mix, and place it in a boiling water bath until a clear solution is obtained. [NOTE-This may take 3 min to 1 h.] When a clear solution is obtained, allow to cool to room temperature. Dry the exterior of the tube, and weigh to the nearest 0.1 mg. Add 0.05 mL of Internal standard solution, and weigh to the nearest 0.1 mg.
Determine the mass of the Internal standard solution added. Mix thoroughly.
Nuclear magnetic resonance spectrometry
(See Nuclear Magnetic Resonance Spectroscopy (761), Quantitative Applications.)
Apparatus: FT-NMR spectrometer at minimum 300 MHz
Acquisition of H NMR spectra: The following parameters may be used.
Sweep width: 8 ppm (about-1.0 to +7 ppm)
Irradiation frequency offset: None
Time domain: NLT 64 K
Pulse width: 90 degree
Pulse delay: 10 s
Dummy scans: 0
Number of scans: 8
Use the CH, signal of the internal standard for shift referencing. Set the shift of the peak of the singlet to 0 ppm. Record the FID signal.
Analysis
Samples: Internal standard solution and Sample solution
Call the integration sub-routine after phase corrections and baseline correction between -0.5 and +6 ppm.
Measure the peak areas of the doublet from the methyl groups of the hydroxypropyl function at +1.2 ppm (A₂), and of the methyl groups
at 0 ppm of the internal standard (A,) without 13C-satellites.
Measure the signal coming from the 3 protons of the methyl group in the hydroxypropyl function.
Calculate the content of hydroxypropyl groups as a percentage (w/w, dried basis):
Result = (N × A2 /A1 ) × (Ci × Wi /W) × (Mr2 /Mr1 ) × [100/(100 − B)] × 100
N = numerical value representing the 3 methyl groups in the internal standard (sodium 3-trimethylsilyl-1-propane sulfonate), 3
A2 = area of the methyl groups of hydroxypropyl in Hydroxypropyl Pea Starch
A1 = area of the methyl groups in the internal standard (sodium 3-trimethylsilyl-1-propane sulfonate)
Ci = concentration of the internal standard in the Internal standard solution (mg/g)
Wi = weight of the Internal standard solution in the NMR tube (g)
W = weight of the washed and dried Hydroxypropyl Pea Starch in the NMR tube (mg)
Mr2 = molecular weight of the internal standard, 218.32 g/mol
Mr1 = molar mass of hydroxypropyl group, 59.09 g/mol
B = moisture content of the washed and dried Hydroxypropyl Pea Starch used in the Sample solution, as a percentage (w/w)
Acceptance criteria: The content of hydroxypropyl groups is 2.0%–7.0%.
4 IMPURITIES
4.1 Inorganic Impurities
Residue on Ignition 〈281〉: NMT 0.6%, determined on a 1.0-g test specimen
Change to read:
4.2 Limit of Iron
Standard iron stock solution: Prepare a solution containing the equivalent of 10 µg/mL of iron, as directed under Iron (241), Procedures.
Procedure 1A (CN 1-Jun-2023).
Diluted standard iron solution: Immediately before use, dilute an accurately measured volume of Standard iron stock solution quantitatively with water to obtain a solution containing the equivalent of 1 µg/mL of iron.
Standard solution: Transfer 10 mL of the Diluted standard iron solution to a test tube. Add 2 mL of citric acid solution (2 in 10) and 0.1 mL of thioglycolic acid, and mix. Add 10 N ammonium hydroxide until the solution is distinctly alkaline to litmus, dilute with water to 20 mL, and mix.
Sample solution: Shake 1.0 g of Hydroxypropyl Pea Starch with 50 mL of 2 N hydrochloric acid, and filter. Transfer 10 mL of the filtrate to a test tube. Add 2 mL of citric acid solution (2 in 10) and 0.1 mL of thioglycolic acid, and mix. Add 10 N ammonium hydroxide until the solution is distinctly alkaline to litmus, dilute with water to 20 mL, and mix.
Acceptance criteria: After 5 min, any pink color in the Sample solution is not more intense than that in the Standard solution, corresponding to a limit of 50 µg/g of iron.
LIMIT OF SULFUR DIOXIDE, Method IV (525): NMT 50 ppm
4.3 Organic Impurities
PROCEDURE 1: LIMIT OF OXIDIZING SUBSTANCES
Sample: 4.0 g of Hydroxypropyl Pea Starch
Analysis: Transfer the Sample to a glass-stoppered, 125-mL conical flask, and add 50.0 mL of water. Insert the stopper, and swirl for 5 min.
Transfer to a glass-stoppered, 50-mL centrifuge tube, and centrifuge to clarify. Transfer 30.0 mL of the clear supernatant to a glass-stoppered, 125-mL conical flask. Add 1 mL of glacial acetic acid and 0.5-1.0 g of potassium iodide. Insert the stopper, swirl, and allow to stand for 25-30 min in the dark. Add 1 mL of starch TS, and titrate with 0.002 N sodium thiosulfate VS to the disappearance of the starch-iodine color. Perform a blank determination, and make any necessary correction. Each mL of 0.002 N sodium thiosulfate VS is equivalent to 34 µg of oxidant, calculated as Hydrogen peroxide.
Acceptance criteria: NMT 1.4 mL of 0.002 N sodium thiosulfate VS is required (20 µg/g, calculated as H2O2).
PROCEDURE 2: FOREIGN MATTER
Sample: 50 mg/mL of Hydroxypropyl Pea Starch in a mixture of glycerin and water (1:1)
Analysis: Examine under a microscope, using NLT 20x magnification and a mixture of glycerin and water (1:1) as a mounting agent.
Acceptance criteria: NMT traces of matter other than Hydroxypropyl Pea Starch granules are present
5 SPECIFIC TESTS
MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62); The total aerobic microbial count does not exceed 103 cfu/g, the total combined molds and yeasts count does not exceed 102 cfu/g, and it meets the requirements of the test for the absence of Escherichia coli.
PH (791)
Sample solution: Suspend 5.0 g of Hydroxypropyl Pea Starch in 25.0 mL of carbon dioxide-free water, and shake for 60 s. Allow to stand for 15 min.
Acceptance criteria: 4.5-8.0
LOSS ON DRYING (731): Dry about 1 g at 130 for 90 min: it loses NMT 15.0% of its weight.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. Store at room temperature.


