Hydroxocobalamin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
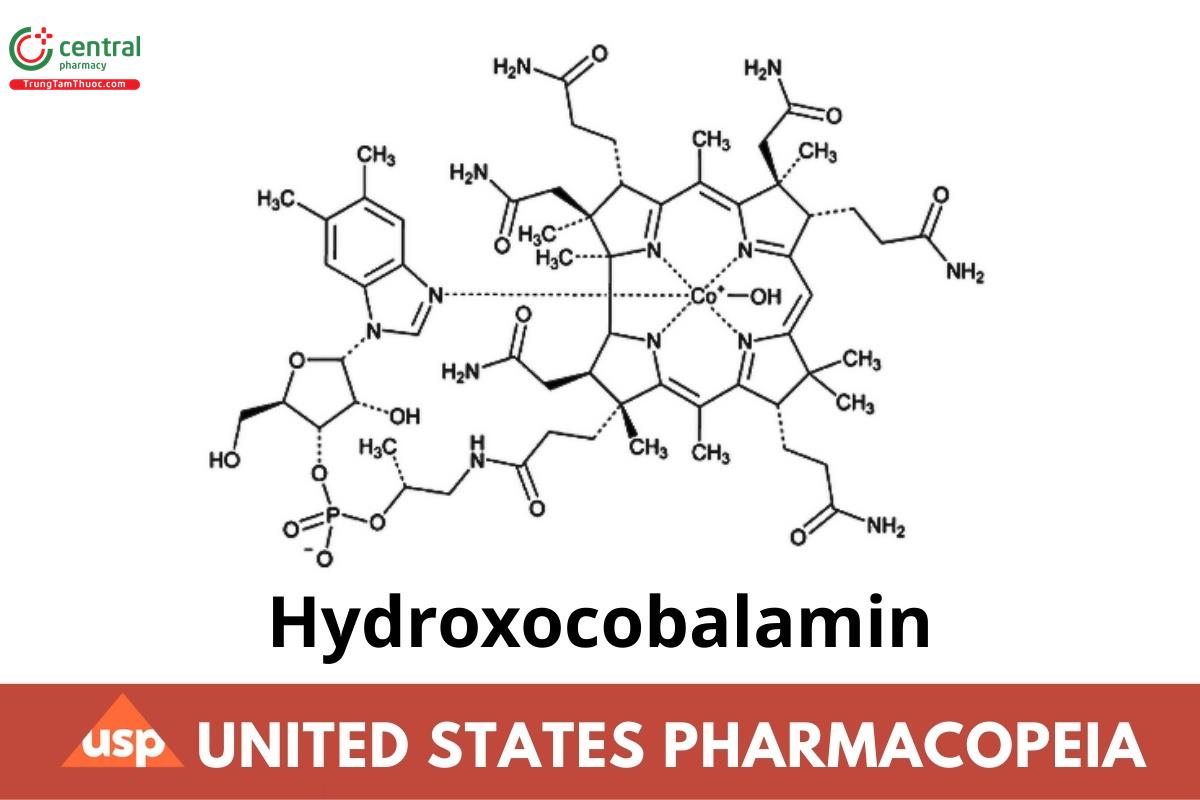
C62H89CoN13O15P 1346.36
Cobinamide, dihydroxide, dihydrogen phosphate (ester), mono(inner salt), 3′-ester with 5,6-dimethyl-1-α-d-ribofuranosyl-1H-benzimidazole;
Cobinamide dihydroxide dihydrogen phosphate (ester), mono(inner salt), 3′-ester with 5,6-dimethyl-1-α-d-ribofuranosylbenzimidazole
CAS RN®: 13422-51-0; UNII: Q40X8H422O.
1 DEFINITION
Hydroxocobalamin contains NLT 95% and NMT 102.0% of hydroxocobalamin (C62H89CoN13O15P), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B. COBALT
Sample: 1 mg of Hydroxocobalamin
Analysis: Fuse the Sample with 50 mg of potassium pyrosulfate in a porcelain crucible. Cool, break up the mass with a glass rod, add 3 mL of water, and boil until dissolved. Add 1 drop of phenolphthalein TS, and add 2 N sodium hydroxide dropwise until a pink color appears. Add 0.5 g of sodium acetate, 0.5 mL of 1 N acetic acid, and 0.5 mL of a 10-mg/mL solution of nitroso R salt. Add 0.5 mL of hydrochloric acid, and boil for 1 min.
Acceptance criteria: A red or orange-red color appears immediately after the addition of nitroso R salt. The red or orange-red color persists after boiling with the addition of hydrochloric acid.
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Buffer solution: 15.6 g/L of sodium dihydrogen phosphate in water. Adjust with phosphoric acid (1 in 100) to a pH of 3.0.
Mobile phase: See Table 1.
Table 1
| Time (min) | Methanol (%) | Buffer solution (%) | Water (%) |
| 0 | 8 | 10 | 82 |
| 20 | 8 | 10 | 82 |
| 40 | 40 | 10 | 50 |
| 45 | 8 | 10 | 82 |
| 50 | 8 | 10 | 82 |
Diluent: Methanol, Buffer solution, and water (8:10:82)
Standard solution: 0.1 mg/mL of USP Hydroxocobalamin Chloride RS in Diluent. Mix to homogenize.
Sample solution: 0.1 mg/mL of Hydroxocobalamin in Diluent. Mix to homogenize.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 351 nm
Column: 4.6-mm × 10-cm and 4.6-mm × 10-cm placed in series; packing L11
Column temperature: 30°
Flow rate: 2.0 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
[Note—The retention time of hydroxocobalamin is about 16 min.]
Suitability requirements
Tailing factor: NMT 2.5
Relative standard deviation: NMT 1.0% from replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of hydroxocobalamin (C62H89CoN13O15P) in the portion of Hydroxocobalamin taken:
Result = (rU/rS) × (CS/ CU) × (MU/MS) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
Cₛ = concentration of USP Hydroxocobalamin Chloride RS in the Standard solution (mg/mL)
CU = concentration of Hydroxocobalamin in the Sample solution (mg/mL)
MU = molecular weight of hydroxocobalamin, 1346.4
MS = molecular weight of hydroxocobalamin chloride, 1382.8
Acceptance criteria: 95%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
4.1 RELATED COMPOUNDS
Buffer solution, Mobile phase, Diluent, and Chromatographic system: Proceed as directed in Assay.
System suitability solution: 0.75 mg/mL of USP Hydroxocobalamin Chloride RS in Diluent
Solution for quantitation: 7.5 µg/mL of USP Hydroxocobalamin Chloride RS in Diluent, diluted from the System suitability solution
Solution for the disregard limit: 0.75 µg/mL of USP Hydroxocobalamin Chloride RS in Diluent, diluted from the Solution for quantitation
Sample solution: 0.75 mg/mL of Hydroxocobalamin in Diluent
System suitability
Sample: System suitability solution
Suitability requirement
Peak-to-valley ratio:2 NLT 2.0 between hydroxocobalamin and its closest impurity
Analysis
Sample: Sample solution
Measure the peak responses of the Sample solution. Disregard the peaks with a response smaller than 0.7 times that of the peak of the Solution for the disregard limit.
Calculate the percentage of each impurity in the portion of Hydroxocobalamin taken:
Result = (rU/rS) × (CS/CU) × (1/F) × (Mr1/Mr2) × 100
rU = peak response of each individual impurity from the Sample solution
rS = peak response from the Solution for quantitation
CS = concentration of USP Hydroxocobalamin Chloride RS in the Solution for quantitation
CU = concentration of Hydroxocobalamin in the Sample solution
F = response factor, 0.8 for cyanocobalamin and 1.0 for any other impurity
Mr1 = molecular weight of hydroxocobalamin, 1346.4
Mr2 = molecular weight of hydroxocobalamin chloride, 1382.8
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| B6-Hydroxymethyl derivative | 0.45 | 0.50 |
| B5-Hydroxymethyl derivative | 0.53 | 0.40 |
| Impurity | 0.70 | 0.30 |
| Impurity | 0.75 | 0.40 |
| Impurity | 0.82 | 0.40 |
| Impurity | 0.93 | 1.1 |
| C8 Epimer | 1.25 | 1.0 |
| Impurity | 1.37-1.51 | 0.50 |
| Cyanocobalamin | 1.90 | 1.0 |
| Any other impurity | - | 0.20 |
| Sum of all impurities | - | 4.0 |
4.2 RESIDUAL SOLVENTS (467)
Acceptance criteria
Acetone: NMT 0.5%.
[Note-For Acceptance criteria for any other residual solvents, see the chapter.]
5 SPECIFIC TESTS
PH (791)
Sample solution: 20 mg/mL of solution
Acceptance criteria: 8.0-10.0
WATER DETERMINATION, Method la(921): 14.0%~18.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers, and store in a cool, dry place. Do not freeze.
USP REFERENCE STANDARDS (11)
USP Hydroxocobalamin Chloride RS
1 Chromolith columns (Merck) are based on a reversed phase employing monolithic C-bonded silica with bimodal pore structure (macropore 2 µm; mesopore 13 nm). Example: Merck Chromolith Performance RP-18 endcapped, 4.6-mm × 10-cm, product #1021290001.
2
Peak-to-valley ratio:
p/v = Hp/Hv
p/v = peak-to-valley ratio
Hp = height above the extrapolated baseline of the minor peak
Hv = height above the extrapolated baseline at the lowest point of the curve separating the minor and major peaks
(See Chromatography 〈621〉, Peak-to-Valley Ratio.) NLT 2.0 between hydroxocobalamin and its closest impurity


