Homatropine Hydrobromide
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
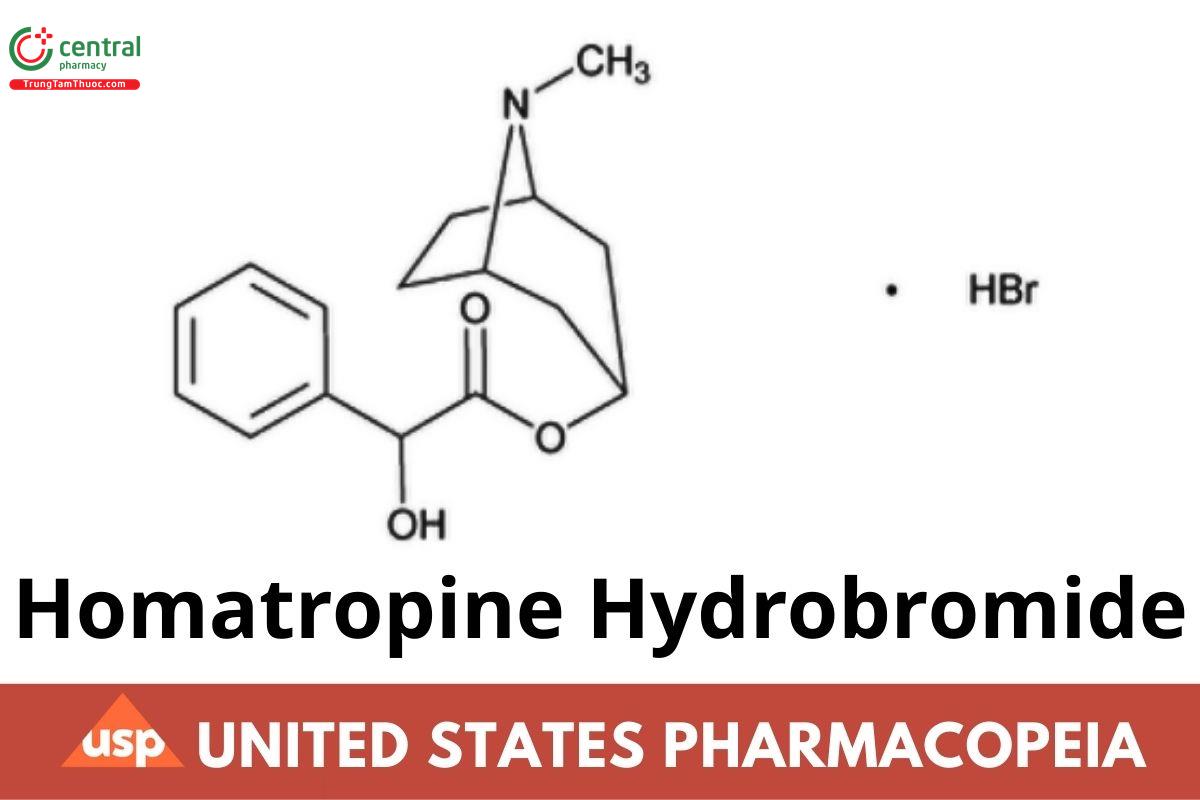
C16H21NO3 · HBr 356.26 (ERR 1-Jul-2022)
Benzeneacetic acid, α-hydroxy-, 8-methyl-8-azabicyclo- [3.2.1]oct-3-yl ester, hydrobromide, endo-(±)-.
(1R,3r,5S)-8-Methyl-8-azabicyclo[3.2.1]octan-3-yl 2-hydroxy-2-phenylacetate hydrobromide (ERR 1-Jul-2022) CAS RN®: 51-56-9; UNII: BEW7469QZ0.
Homatropine Hydrobromide contains not less than 98.0 percent and not more than 102.0 percent of C16H21NO3 · HBr, calculated on the dried basis.
Packaging and storage—Preserve in tight, light-resistant containers.
USP Reference standards 〈11〉—
USP Homatropine Hydrobromide RS
USP Scopolamine Hydrobromide RS
Identification—
A: Spectroscopic Identication Tests 〈197〉, Infrared Spectroscopy: 197K.
B: It responds to the tests for Bromide 〈191〉.
pH 〈791〉: between 5.7 and 7.0, in a solution (1 in 50).
Loss on drying 〈731〉—Dry it at 105° for 2 hours: it loses not more than 1.5% of its weight.
Residue on ignition 〈281〉: not more than 0.25%.
Limit of tropine—
Adsorbent: 0.2-mm layer of chromatographic silica gel mixture.
Diluent—Prepare a mixture of methanol and water (9:1).
Test solution—Transfer about 0.2 g of Homatropine Hydrobromide to a 5-mL volumetric ask, dissolve in and dilute with Diluent to volume, and mix.
Standard solution—Dilute 0.5 mL of the Test solution with Diluent to 100.0 mL.
Tropine reference solution—Prepare a solution of tropine having a concentration of about 0.4 mg per mL in Diluent. Application volume: 1 µL.
Developing solvent system: a mixture of ethyl acetate, anhydrous formic acid, and water (67: 16.5: 16.5).
Procedure—Proceed as directed for Thin-Layer Chromatography under Chromatography 〈621〉, applying the Test solution, the Standard solution, and the Tropine reference solution. Spray the plate with Dragendorff's reagent, followed by Hydrogen peroxide TS, and immediately cover with a glass plate of the same size. Examine the plate no later than 5 to 10 minutes after spraying. In the chromatogram obtained from the Test solution, identify the spot corresponding to the principal spot in the chromatogram of the Tropine reference solution: this spot is not more intense than the spot obtained from the Standard solution: not more than 0.5% of tropine is found.
Chromatographic purity—
Buffer solution, Mobile phase, System suitability solution, and Chromatographic system—Proceed as directed in the Assay. Standard solution—Use the Standard preparation, prepared as directed in the Assay.
Test solution—Use the Assay preparation, prepared as directed in the Assay.
Procedure—Separately inject a volume (about 7 µL) of the Test solution into the chromatograph, record the chromatogram, and measure the responses for the major peaks. Continue the elution for 2.2 times the retention time of the homatropine peak. Disregard the peak for the bromide ion, which appears close to the solvent peak. Calculate the percentage of each impurity in the portion of Homatropine Hydrobromide taken by the formula:
100(ri /rs )
in which ri and rs are the peak response for each impurity and the sum of all peak responses, respectively, obtained from the Test solution. In dition to not exceeding the limits for each impurity in Table 1, not more than 0.1% of any other individual impurity is found; and not more than 1.0% of total impurities is found.
Table 1
Impurity | Relative Retention Time | Limit (%) |
Mandelic acid | 0.3 | 0.1 |
Dehydrohomatropine | 0.9 | 0.5 |
Scopolamine | 1.1 | 0.1 |
Atropine | 1.9 | 0.1 |
Assay—
Buffer solution—Dissolve 6.8 g of monobasic potassium phosphate and 7.0 g of sodium 1-heptanesulfonate monohydrate in 1000 mL of water, adjust with 3 M phosphoric acid to a pH of 2.7, and mix.
Mobile phase—Prepare a filtered and degassed mixture of Buffer solution and methanol (67:33).
Standard preparation—Dissolve an accurately weighed quantity of USP Homatropine Hydrobromide RS in Mobile phase to obtain a solution having a concentration of about 2 mg per mL.
System suitability solution—Prepare a solution of USP Scopolamine Hydrobromide RS having a concentration of about 0.1 mg per mL. Transfer 10 mL of this solution to a 100-mL volumetric flask, add 0.5 mL of the Standard preparation, and dilute with Mobile phase to volume. Assay preparation—Transfer about 100 mg of Homatropine Hydrobromide, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography 〈621〉)—The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm × 10-cm column that contains 3-µm packing L1. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 40°. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 1.0%. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution between homatropine and scopolamine peaks is not less than 1.5. Procedure—Separately inject equal volumes (about 7 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C16H21NO3 · HBr in the portion of Homatropine Hydrobromide taken by the formula:
50C(rU /rS )
in which C is the concentration, in mg per mL, of USP Homatropine Hydrobromide RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.


