Halothane
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
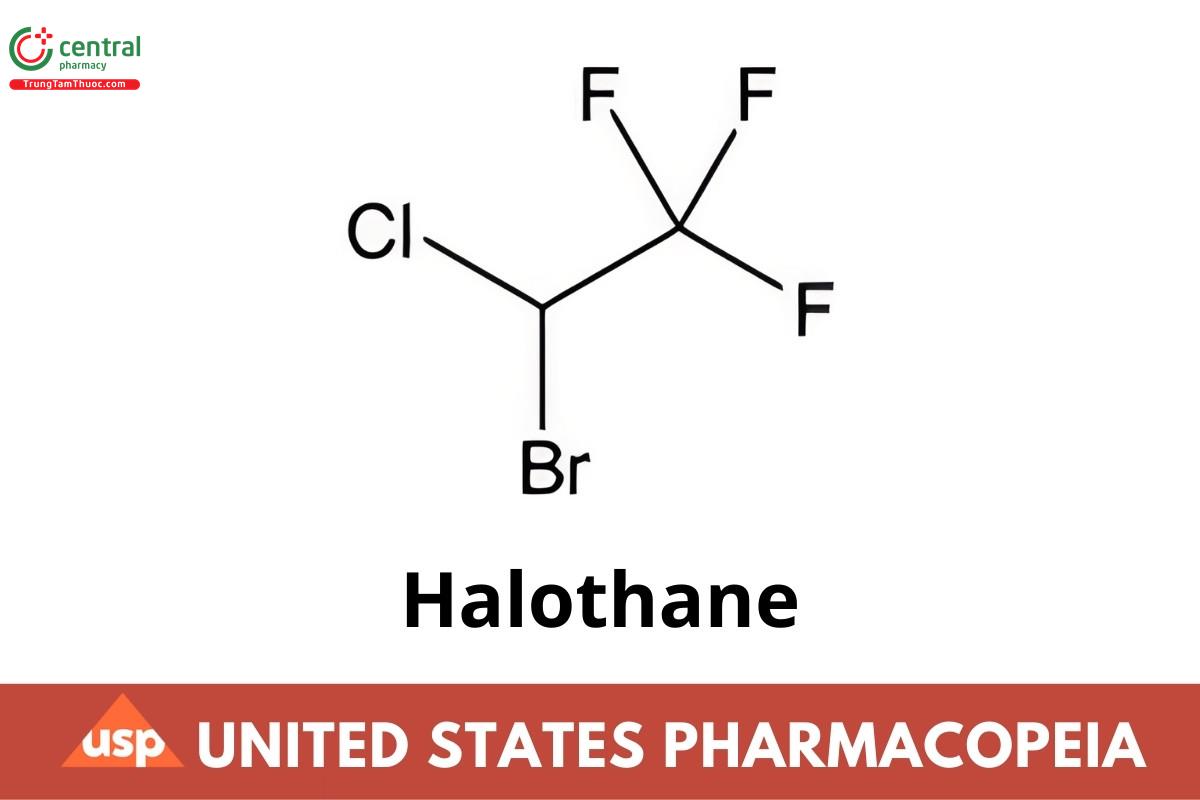
C2HBrCIF3 197.38
Ethane, 2-bromo-2-chloro-1,1,1-trifluoro-, (±)-;
(±)-2-Bromo-2-chloro-1,1,1-trifluoroethane CAS RN®: 151-67-7; UNII: UQT9G45D1P.
1 DEFINITION
Halothane contains NLT 0.008% and NMT 0.012% of thymol, by weight, as a stabilizer.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 1975 (CN 1 -MAY-2020)
Sample solution: 1 in 25
Medium: Carbon disulfide
Acceptance criteria: Meets the requirements
3 OTHER COMPONENTS
THYMOL CONTENT
Buffer: Use pH 8.0 Alkaline Borate Buffer (see Reagents, Indicators, and Solutions-Solutions).
Chlorimide solution: 4 mg/mL of 2,6-dibromoquinonechlorimide in dehydrated alcohol. [NOTE-Prepare a fresh solution for each assay.]
Standard thymol solution: 0.1 mg/mL of thymol in 0.25 N sodium hydroxide
Standard solution A: Pipet 1 mL of Standard thymol solution into a 100-mL volumetric flask, and add 0.25 N sodium hydroxide to make the final volume 5 mL. Add 10 mL of Buffer, mix by gentle swirling, and add 1 mL of Chlorimide solution. Allow to stand for 15 min, accurately timed. Add 3 mL of 0.25 N sodium hydroxide, and dilute with water to volume.
Standard solution B: Pipet 3 mL of Standard thymol solution into a 100-mL volumetric flask. Proceed as directed for Standard solution A, beginning with "add 0.25 N sodium hydroxide...".
Standard solution C: Pipet 5 mL of Standard thymol solution into a 100-mL volumetric flask. Proceed as directed for Standard solution A, beginning with "Add 10 mL of Buffer...".
Sample solution: Place 2 mL of Halothane in a 100-mL volumetric flask containing 5 mL of 0.25 N sodium hydroxide, and mix by gentle swirling. Evaporate the halothane under a stream of nitrogen, and add 10 mL of Buffer and 1 mL of Chlorimide solution. Swirl gently, and allow to stand for 15 min, accurately timed. Add 3 mL of 0.25 N sodium hydroxide, and add water to volume.
Blank solution: Pipet 5.0 mL of 0.25 N sodium hydroxide into a 100-mL volumetric flask. Add 10 mL of Buffer, mix by gentle swirling, and add 1 mL of Chlorimide solution. Allow to stand for 15 min, accurately timed. Add 3 mL of 0.25 N sodium hydroxide, and dilute with water to volume.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).)
Mode: Vis
Analytical wavelength: 590 nm
Analysis
Samples: Standard solution A, Standard solution B, Standard solution C, Sample solution, and Blank solution
Measure the absorbances of Standard solution A, Standard solution B, and Standard solution C relative to the Blank solution. Plot the readings, and draw the curve of best fit. Read the absorbance of the Sample solution, and by reference to the Standard thymol curve, calculate the percentage of thymol in the weight of Halothane taken.
Acceptance criteria: 0.008%-0.012%, by weight
4 IMPURITIES
4.1 LIMIT OF NONVOLATILE RESIDUE
Analysis: Evaporate 50 mL in a tared dish on a steam bath to dryness, dry the residue at 105° for 2 h, and weigh.
Acceptance criteria: NMT 1 mg of the residue remains.
4.2 CHLORIDE AND BROMIDE
Analysis: Shake 25 mL with 25 mL of water for 5 min, and allow the liquids to separate completely. Draw off the water layer, and to 10 mL add 1 drop of nitric acid and 5 drops of silver nitrate TS.
Acceptance criteria: No opalescence is produced.
4.3 ORGANIC IMPURITIES
Standard solution: Add 1.0 µL of 1,1,2-trichloro-1,2,2-trifluoroethane to 20.0 mL of Halothane.
Sample solution: Halothane
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 3-m x 2-mm stainless steel; 20% G24 on support S1AB
Temperatures
Column: 60°
Injection port: 200°
Detector: 200°
Carrier gas: Nitrogen
Flow rate: 15 mL/min
Injection volume: 2 µL
System suitability
Sample: Standard solution
[NOTE-The retention times for 1,1,2-trichloro-1,2,2-trifluoroethane and halothane are 5 and 13 min, respectively.]
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: 0.005%; the total area of all peaks (except that of halothane) recorded from the Sample solution does not exceed that due to the added 1,1,2-trichloro-1,2,2-trifluoroethane in the Standard solution.
5 SPECIFIC TESTS
5.1 ACIDITY OR ALKALINITY
Analysis: Shake 20 mL with 20 mL of carbon dioxide-free water for 3 min, and allow the layers to separate.
Acceptance criteria: The aqueous layer requires NMT 0.1 mL of 0.010 N sodium hydroxide or NMT 0.6 mL of 0.010 N hydrochloric acid for neutralization, using bromocresol purple TS as the indicator.
5.2 DISTILLING RANGE, Method II(721)
NLT 95% distills within a 1° range between 49° and 51°, and NLT 100% distills between 49° and 51°, a correction factor of 0.040°/mm being applied as necessary.
5.3 REFRACTIVE INDEX (831)
1.369-1.371 at 20°
5.4 SPECIFIC GRAVITY (841)
1.872-1.877 at 20°
5.5 WATER DETERMINATION, Method I(921)
NMT 0.03%
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in tight, light-resistant containers, preferably of Type NP glass, and avoid exposure to excessive heat. Dispense it only in the original container.
6.2 USP REFERENCE STANDARDS (11)
USP Halothane RS


