Halazone
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
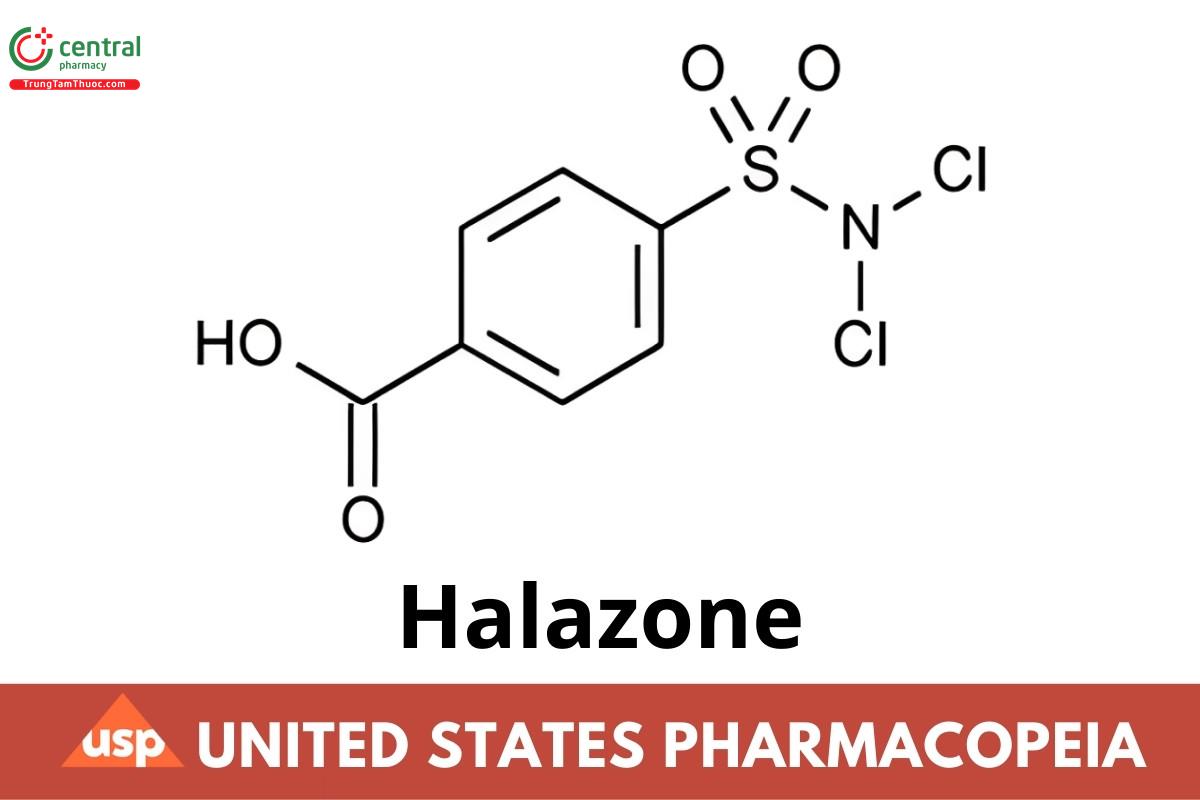
C7H5CI2NO4S 270.09
Benzoic acid, 4-[(dichloroamino)sulfonyl]-;
p-(Dichlorosulfamoyl)benzoic acid CAS RN®: 80-13-7; UNII: G359OL82VB.
1 DEFINITION
Halazone contains NLT 91.5% and NMT 100.5% of C7H5CI2NO4S, calculated on the dried basis.
2 IDENTIFICATION
PROCEDURE
Sample: 100 mg
Analysis: Add the Sample to 5 mL of a sodium bromide solution (1 in 10).
Acceptance criteria: Bromine is liberated from the mixture.
3 ASSAY
PROCEDURE
Sample: 150 mg
Analysis: Add the Sample to 10 mL of 2.5 N sodium hydroxide in a 250-mL iodine flask, and stir well to dissolve. Add 75 mL of water, then promptly add 15 mL of potassium iodide solution (1 in 10), and mix. Acidify with 10 mL of 6 N acetic acid, and titrate immediately with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium thiosulfate is equivalent to 6.752 mg of C7H5CI2NO4S.
Acceptance criteria: 91.5%-100.5% on the dried basis
4 IMPURITIES
ORGANIC IMPURITIES
PROCEDURE: READILY CARBONIZABLE SUBSTANCES TEST (271)
Sample: 100 mg
Analysis: Dissolve the Sample in 0.5 mL of sulfuric acid.
Acceptance criteria: No blackening occurs, although some effervescence may take place.
5 SPECIFIC TESTS
LOSS ON DRYING (731): Dry a sample over phosphorus pentoxide for 4 h: it loses NMT 0.5% of its weight.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.


