Guar Gum
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
CAS RN®: 9000-30-0.
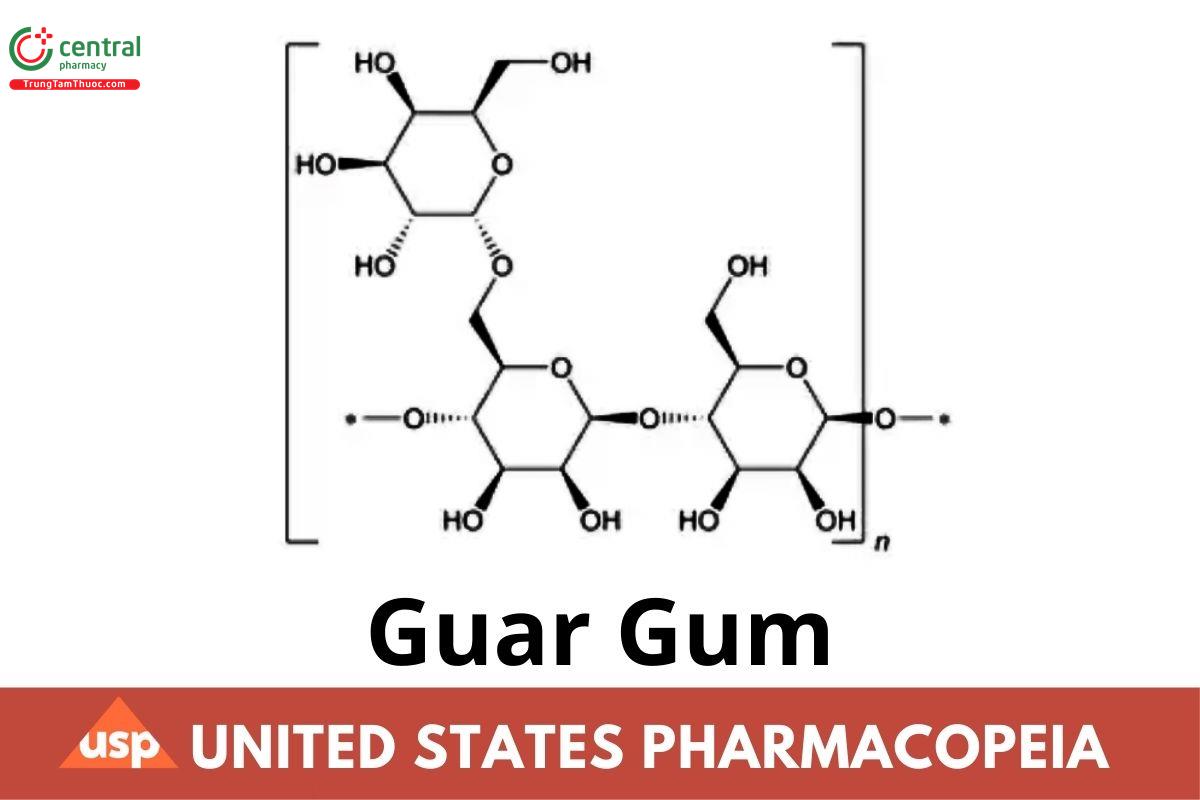
1 DEFINITION
Guar Gum is the flour obtained by grinding the endosperms of seeds of Cyamopsis tetragonolobus (L.) Taub. (Fam. Leguminosae). It consists chiefly of high molecular weight hydrocolloidal polysaccharides composed of galactomannan. The content of galactomannan is NLT 66.0%. Galactomannan consists of a linear main chain of β-(1→4)-glycosidically linked mannopyranoses and single α-(1→6)-glycosidically linked galactopyranoses, and the ratio of the mannose and galactose is from 1.4:1 to 2.2:1.
2 IDENTIFICATION
Indication for a Polymeric Compound and Distinction from Locust Bean Gum
Sample: 2 g
Analysis 1: Place the Sample in a 400-mL beaker, and moisten it with 4 mL of isopropyl alcohol. Add 200 mL of cold water with vigorous stirring, and continue stirring until the Sample is completely and uniformly dispersed.
Acceptance criteria 1: An opalescent, viscous dispersion results.
Analysis 2: Transfer 100 mL of the sample dispersion prepared above to a 400-mL beaker, heat in a boiling water bath for about 10 min, and then cool to room temperature.
Acceptance criteria 2: No appreciable increase in viscosity is produced (distinction from locust bean gum: see Reagents, Indicators, and Solutions—Reagent Specications).
Identification of Constituting Mannose and Galactose by Thin-Layer Chromatography
Mobile phase: Acetonitrile and water (85:15)
Standard solution: Dissolve 10 mg of USP Galactose RS and 10 mg of USP Mannose RS in 2 mL of water, and dilute with methanol to 20 mL. Sample solution: Transfer 20 mg of Guar Gum to a test tube, add 4 mL of a 100 mg/mL solution of trifluoroacetic acid, and shake vigorously to dissolve the forming gel. Stopper the tube, and heat the mixture at 115° for 1 h 20 min in a dry bath (heating block) or oil bath. Cool, transfer the hydrolysate to a centrifuge tube, and centrifuge. Some suspended particles/gel are formed. Pass the supernatant solution through a 0.45-µm disc filter. Wash the test tube and the centrifuge tube with two 5-mL portions of water, and filter. Combine the washing filtrate with the filtered supernatant of the hydrolysate. Transfer the combined clear filtrate to a 50-mL flask, and evaporate the solution to dryness under reduced pressure. To the resulting residue add 0.2 mL of water and 1.8 mL of methanol.
Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Mode: TLC
Absorbent layer: 0.25-mm silica gel 60 F254
Application volume: 5 µL, as 9-mm bands, using an automated apparatus
Spray reagent: Dissolve 3 g of phthalic acid and 0.3 g of aminohippuric acid in ethyl alcohol, and dilute with ethyl alcohol to 100 mL.
Analysis
Samples: Standard solution and Sample solution
Develop over a path of 15 cm. Spray with Spray reagent, and dry at 120° for 5 min.
Acceptance criteria: The chromatogram from the Standard solution shows, in the lower region, two clearly separated brownish or yellowish zones due to galactose and mannose in order of increasing R value. The chromatogram from the Sample solution shows two zones due to F galactose and mannose.
3 ASSAY
Content of Galactomannan and Ratio of Constituting Mannose and Galactose
Mobile phase: Water
System suitability solution: 5 mg/mL of USP Galactose RS, 5 mg/mL of USP Mannose RS, 5 mg/mL of USP Xylose RS, and 5 mg/mL of USP Dextrose RS in Mobile phase
Standard solution: 10 mg/mL of USP Galactose RS and 10 mg/mL of USP Mannose RS in Mobile phase
Sample solution A: Transfer 100 mg of Guar Gum to a glass test tube. Add 2.0 mL of water and 2.0 mL of 1 M trifluoroacetic acid to the tube, and mix on a vortex mixer for 30 s. Incubate the solution at 105° in an oil-bath heating module for 6 h. After the first 15 min of incubation, mix on a vortex mixer for 30 s. After the 30 min of incubation, mix on a vortex mixer for 30 s. [Note—This ensures that Guar Gum does not stick to the bottom of the test tube and burn.] Before HPLC analysis, mix on a vortex mixer for 30 s, and pass the solution through a 0.45- µm PES (polyethersulfone) membrane syringe filter.
Sample solution B: 5 mg/mL of Guar Gum in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Refractive index
Column: 8.0-mm × 30-cm; 7-µm packing L22
Temperatures
Detector: 55°
Column: 80°
Flow rate: 0.75 mL/min
Injection volume: 10 µL
Detector purge time: 1 min
Run time: 17 min
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for Glucose, xylose, galactose, and mannose are 0.88, 0.94, 1.00, and 1.10, respectively.] Suitability requirements
Resolution: NLT 0.9 between dextrose and xylose; NLT 1.0 between xylose and galactose; NLT 1.5 between galactose and mannose, System suitability solution
Tailing factor: 0.8–1.8 for the galactose and mannose peaks, Standard solution
Relative standard deviation: NMT 2.0% for the galactose and mannose peaks, Standard solution
Analysis
Samples: Standard solution, Sample solution A, and Sample solution B
In the chromatogram of Sample solution B, no galactose and mannose peaks are observed.
Calculate the percentage of galactose (CG) or mannose (CM) in the portion of Guar Gum taken:
Result (CG or CM) = (rU/rS) × (CS/CU) × 100
rU = peak response of galactose or mannose in Sample solution A
rS = peak response of galactose or mannose in the Standard solution
CS = concentration of USP Galactose RS or USP Mannose RS in the Standard solution (mg/mL)
CU = concentration of Guar Gum in Sample solution A (mg/mL)
Calculate the content of galactomannans in the portion of Guar Gum taken:
Result = CM + CG
Calculate the ratio of constituting mannose and galactose in the portion of Guar Gum taken:
Result = CM/CG
Acceptance criteria
Content of galactomannan: NLT 66.0%
Ratio of constituting mannose and galactose: 1.4–2.2
4 IMPURITIES
Change to read:
Arsenic 〈211〉, Procedures, Procedure 2 : NMT 3 µg/g
Change to read:
Lead 〈251〉, Procedures, Procedure 1
Analysis: Prepare a Test Preparation as directed in the chapter, and use 10 mL of Diluted Standard Lead Solution (10 µg of Pb) for the test. Acceptance criteria: NMT 10 µg/g
5 SPECIFIC TESTS
Articles of Botanical Origin, Total Ash 〈561〉: NMT 1.5%
Acid-Insoluble Matter
Sample: 1.5 g
Analysis: Transfer the Sample to a 250-mL beaker containing 150 mL of water and 1.5 mL of sulfuric acid. Cover the beaker with a watch glass, and heat the mixture on a steam bath for 6 h, rubbing down the wall of the beaker frequently with a rubber-tipped stirring rod and replacing any water lost by evaporation. At the end of the 6 h heating period, add 500 mg, accurately weighed, of a filter aid, and pass through a tared, ashless filter. Wash the residue several times with hot water, dry the filter and its contents at 105° for 3 h, cool in a desiccator, and weigh. Determine the amount of acid-insoluble matter by subtracting the weight of the filter aid from that of the residue. Acceptance criteria: NMT 7.0%
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count does not exceed 104 cfu/g, and the total combined molds and yeasts count does not exceed 102 cfu/g. It meets the requirements of the tests for absence of Salmonella species and Escherichia coli. It is recommended that the enrichment broth contain a 1% cellulase solution additive to optimize the recovery of Salmonella from this material.
Protein
Sample: 1.0 g
Analysis: Transfer the Sample to a 500-mL Kjeldahl flask, and proceed as directed in Nitrogen Determination 〈461〉, Method I. Determine the percentage of nitrogen. Calculate the amount of protein by multiplying the percentage of nitrogen by 6.25.
Acceptance criteria: NMT 10.0%
Starch
Analysis: To a dispersion (1 in 10) of Guar Gum add a few drops of iodine TS.
Acceptance criteria: No blue color is produced.
Loss on Drying 〈731〉
Analysis: Dry at 105° for 5 h.
Acceptance criteria: NMT 15.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP Dextrose RS
USP Galactose RS
USP Mannose RS
USP Xylose RS


