Guanidine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
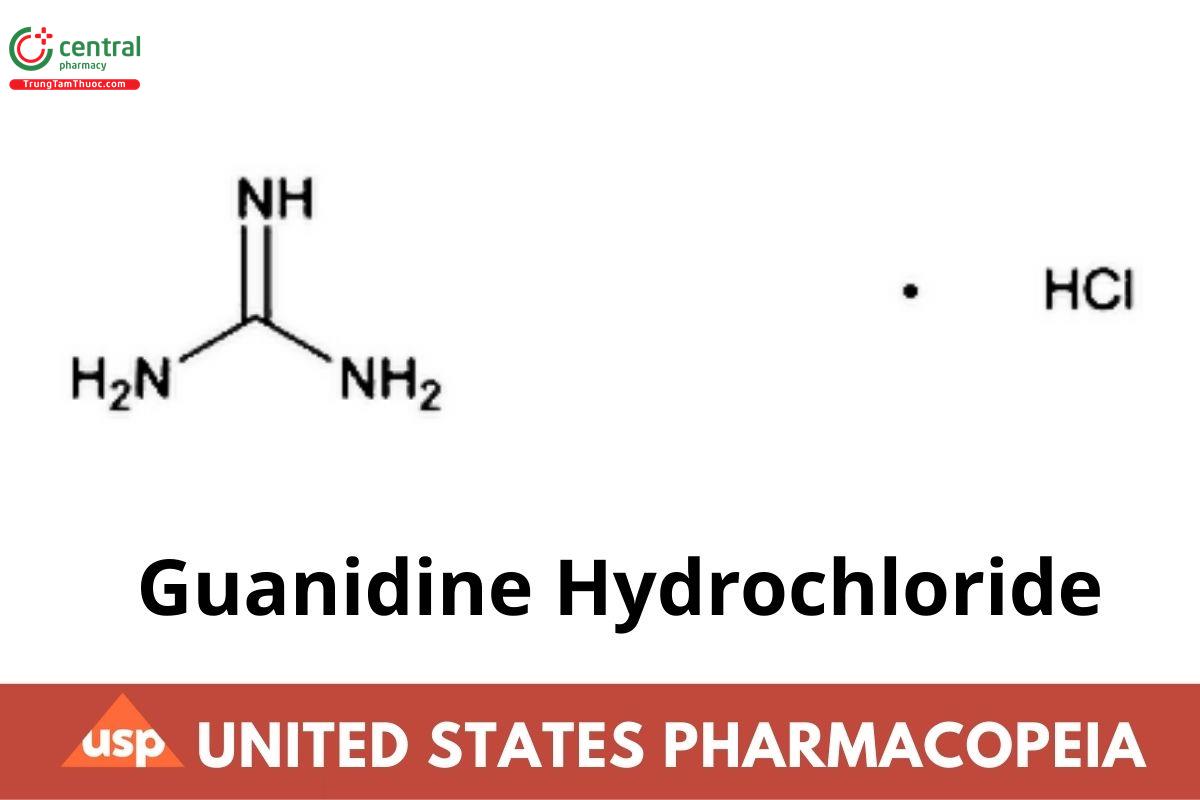
CH5N3 · HCl 95.53
Guanidine hydrochloride (1:1);
Guanidine hydrochloride CAS RN: 50-01-1.
1 DEFINITION
Guanidine Hydrochloride contains NLT 99.5% and NMT 101.0% of guanidine hydrochloride (CH5N3 · HCl), calculated on the dried basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A or 197K
B. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 197U
Sample solution: Equivalent to 572 mg/mL of anhydrous Guanidine Hydrochloride
Analysis: Determine the absorbance of the Sample solution at 230, 260, and 275 nm.
Acceptance criteria
At 230 nm: NMT 0.2
At 260 nm: NMT 0.03
At 275 nm: NMT 0.03
C. IDENTIFICATION TESTS GENERAL (191), Chemical Identification Tests, Chloride: Meets the requirements of test A
3 ASSAY
PROCEDURE
Sample solution: 350 mg, previously dried
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.1 N silver nitrate VS
Endpoint detection: Potentiometric
Blank: Mix 10 mL each of water, glacial acetic acid, and 0.2% polyvinyl alcohol and 100 ml. of methanol.
Analysis: Dissolve the Sample in 10 mL of water, add 10 mL of glacial acetic acid, 10 mL of 0.2% polyvinyl alcohol, and 100 ml of methanol.
Titrate with Titrant to a potentiometric endpoint. Perform a blank determination.
Calculate the percentage of guanidine hydrochloride (CH5N3 · HCl ) in the portion of Guanidine Hydrochloride taken:
Result = [(VS − VB ) × NA × F × 100]/W
VS = volume of Titrant consumed by the Sample (mL)
VB = volume of Titrant consumed by the Blank (mL)
NA = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 95.53 mg/mEq
W = weight of the Sample (mg)
Acceptance criteria: 99.5%–101.0%
4 IMPURITIES
RESIDUE ON IGNITION (281)
Sample: 2.0 g
Analysis: Heat a quartz crucible at 800° for 30 min, allow to cool in a desiccator, and weigh. Evenly distribute the Sample in the crucible and moisten the Sample with 0.5 mL of sulfuric acid. Volatilize the Sample with a Bunsen burner. Place the crucible in the furnace and ignite the residue at 800° for 15 min or until all carbon has been removed. Cool in a desiccator and weigh.
Acceptance criteria: NMT 0.05%
CHLORIDE AND SULFATE (221), Sulfate: A 1.0-g portion shows no more sulfate than corresponds to 0.052 mL of 0.02 N sulfuric acid TS (0.005%).
LIMIT OF NITRATE
Standard nitrate solution and Brucine sulfate solution: Prepare as directed in Reagents, Indicators, and Solutions-Buffer Solutions.
Sample solution: Dissolve 0.40 g in 3.0 mL of water, and add Brucine sulfate solution to make 50 mL.
Control solution: To 2.0 mL of Standard nitrate solution and 1.0 mL of water, add 0.40 g of Guanidine Hydrochloride, then add Brucine sulfate solution to make 50 mL.
Blank: 50.0 mL of Brucine sulfate solution
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).)
Mode: Vis
Analytical wavelength: 410 nm
Cell: 1 cm
Analysis: Heat the Sample solution, Control solution, and Blank in a boiling water bath for 15 min with periodic gentle swirling, then cool rapidly in an ice bath to room temperature. Adjust a suitable spectrophotometer with the Blank to zero absorbance at 410 nm. Determine the absorbance of the Sample solution and the Control solution.
Calculate the percentage of nitrate in the portion of Guanidine Hydrochloride taken:
Result = [Au /(Ac − Au )] × F
Au = absorbance of the Sample solution
Ac = absorbance of the Control solution
F = limit of nitrate, 0.005%
Acceptance criteria: NMT 0.005%
5 SPECIFIC TESTS
5.1 ACIDITY
Sample: 20 g
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.1 N sodium hydroxide VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 200 mL of water, add 0.15 mL of phenolphthalein TS, and titrate with Titrant to a permanent pink endpoint.
Calculate the percentage of hydrochloric acid in the portion of Guanidine Hydrochloride taken:
Result = [(VS × NA × F) × 100/W]
VS = volume of Titrant consumed by the Sample (mL)
NA = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 0.03646 g/mEq
W = weight of the Sample (g)
Acceptance criteria: NMT 0.01%
5.2 WATER-INSOLUBLE SUBSTANCES
[NOTE-If intended for use in preparing parenteral dosage forms, it meets the requirements of the Water-Insoluble Substances test.]
Sample: 50.0 g
Analysis: Dissolve the Sample in 25 mL of ambient temperature water. Use a Teflon-encapsulated magnetic stirring bar and electric stir plate to dissolve the Sample, if necessary. Pass the solution through the tared filtering crucible, previously dried at 105 ± 2° for 1 h. Rinse the sample vessel and crucible filter with 50 mL of water. Dry the crucible at 105 ± 2° for 1 h.
Acceptance criteria: NMT 0.05%
LOSS ON DRYING (731)
Sample: 2.0 g
Analysis: Dry the Sample at 105 ± 2° for 4 h.
Acceptance criteria: NMT 0.5%
MELTING RANGE OR TEMPERATURE (741), Procedures, Procedure for Class I. Apparatus I
Analysis: Proceed as directed in the chapter.
[NOTE-Finely grind with a mortar and pestle and dry over silica gel for NLT 16 h before testing.]
Acceptance criteria: 184°-188°
BACTERIAL ENDOTOXINS TEST (85): If labeled for use in preparing parenteral dosage forms, it also meets the following requirements. The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Guanidine Hydrochloride is used can be met. Where the label states that Guanidine Hydrochloride must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Guanidine Hydrochloride is used can be met.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. Store in a dry place and at a temperature below 30°.
LABELING: Where Guanidine Hydrochloride is intended for use in the manufacture of injectable dosage forms, it is so labeled.
USP REFERENCE STANDARDS (11)
USP Guanidine Hydrochloride RSA (NF 1-Aug-2023)


