Guanabenz Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
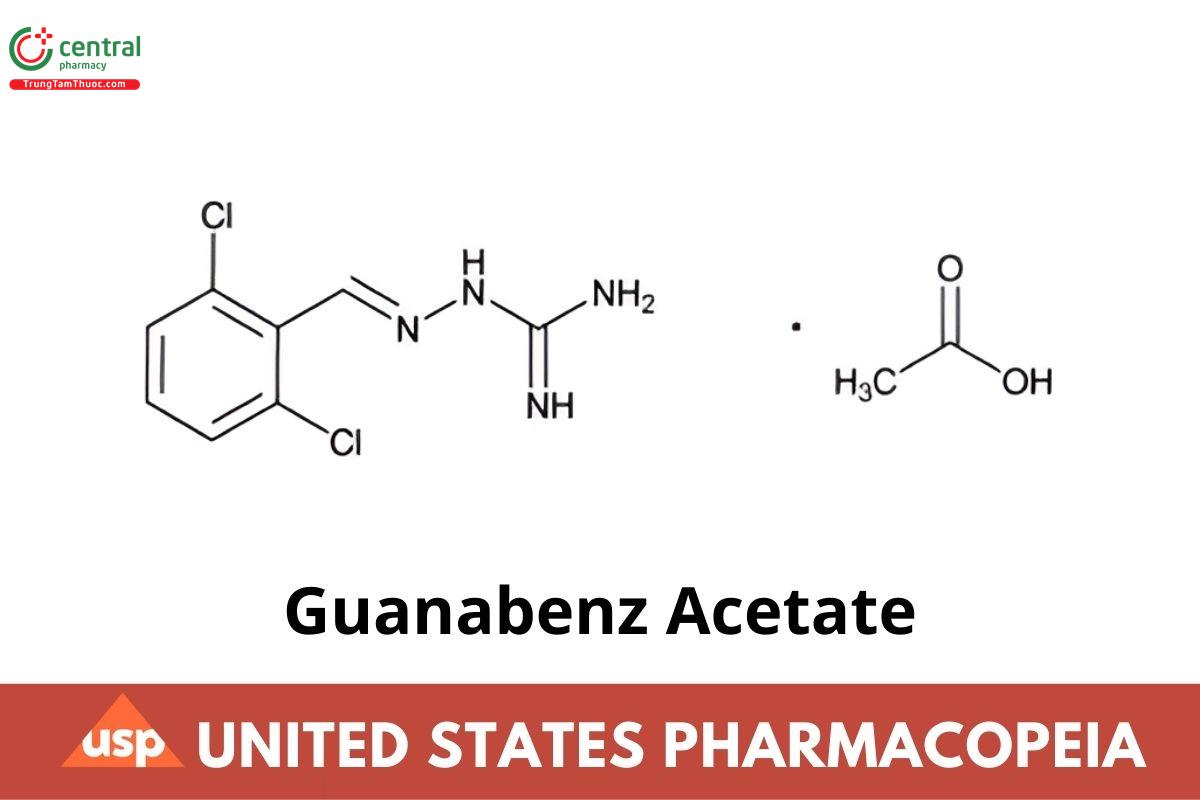
C8H8Cl2N4 · C2H4O2 291.13
Hydrazinecarboximidamide, 2-[(2,6-dichlorophenyl)methylene]-, monoacetate;
[(2,6-Dichlorobenzylidene)amino]guanidine monoacetate CAS RN®: 23256-50-0; UNII: 443O19GK1A.
1 DEFINITION
Guanabenz Acetate contains NLT 98.0% and NMT 101.5% of the labeled amount of guanabenz acetate (C10H12N4O2Cl2).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
3 ASSAY
Procedure
Sample solution: 4 mg/mL of Guanabenz Acetate in glacial acetic acid
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Endpoint detection: Potentiometric
Analysis: Titrate 50 mL of the Sample solution with Titrant. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 29.12 mg of guanabenz acetate (C10H12N4O2Cl2).
Acceptance criteria: 98.0%–101.5%
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.2%
4.1 Organic Impurities
Diluent: A mixture of formic acid and methanol (1 in 2000)
Aminoguanidine bicarbonate solution: Transfer 100 mg of aminoguanidine bicarbonate to a test tube, add 0.05 mL of formic acid, and warm gently to dissolve. Quantitatively transfer the contents of the test tube to a 10-mL volumetric flask, and dilute with methanol to volume.
Standard solution A: 0.1 mg/mL of USP Guanabenz Acetate RS in Diluent prepared as follows. Transfer a suitable amount of USP Guanabenz Acetate RS to a suitable volumetric flask, and dissolve in about 50% of total volume of Diluent. Add 0.1 mL of Aminoguanidine bicarbonate solution per mg of USP Guanabenz Acetate RS, and dilute with Diluent to volume.
Standard solution B: 0.05 mg/mL of USP Guanabenz Acetate RS in Diluent from Standard solution A
Standard solution C: 0.02 mg/mL of USP Guanabenz Acetate RS in Diluent from Standard solution A
Sample solution: 10 mg/mL of Guanabenz Acetate in Diluent
4.1.1 Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 μL
Developing solvent system: Chloroform, methanol, and ammonium hydroxide (60:40:1)
4.1.2 Analysis
Samples: Diluent, Standard solution A, Standard solution B, Standard solution C, and Sample solution
Allow the chromatographic chamber to equilibrate in Developing solvent system for at least 30 min before use. Prewash a plate coated with Adsorbent by placing it in the chromatographic chamber, allowing the solvent front to rise to the top of the plate, dry it in air, and activate it by heating at 105° for 20 min. Within 30 min after preparation, separately apply portions of each of the Standard solutions, the Sample solution, and the Diluent. Allow the spots to dry, and place the plate in the chromatographic chamber. When the solvent has moved about three-fourths of the length of the plate, remove the plate, and allow it to air-dry for about 30 min. Examine the plate under short-wavelength UV light. Estimate the amount of any secondary spots, disregarding spots that have the same R as those from the Diluent, observed in the chromatogram of the Sample solution by comparison with each Standard solution. Place the plate in a chamber saturated with iodine vapors for about 10 min. Remove and examine the plate. Estimate the amount of any spot in the chromatogram of the Sample solution that has an R corresponding to the R of the spot produced by the aminoguanidine bicarbonate by comparison with each Standard solution.
Acceptance criteria: No individual secondary spot is greater in size or intensity than the spot produced by Standard solution B (0.5%), and the total of any such spots observed is NMT 1%.
Change to read:
4.2 Limit of 2,6-Dichlorobenzaldehyde
Internal standard solution 1: 1 mg/mL of p-chlorobenzaldehyde in chloroform
Internal standard solution 2: 0.1 mg/mL of p-chlorobenzaldehyde in chloroform from Internal standard solution 1
Standard stock solution: 1 mg/mL of 2,6-dichlorobenzaldehyde in chloroform
Standard solution: 0.4 mg/mL of 2,6-dichlorobenzaldehyde from the Standard stock solution and 0.1 mg/mL of p-chlorobenzaldehyde from Internal standard solution 1 in chloroform
Sample solution: Transfer 200 mg of Guanabenz Acetate to a 30-mL glass-stoppered centrifuge tube. Add 10 mL of 0.1 N hydrochloric acid, and shake to dissolve. Add 1.0 mL of Internal standard solution 2, and shake. Centrifuge, and transfer a portion of the lower layer to a stoppered container. The lower layer must be removed within 10 min of adding the acid to the centrifuge tube.
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 1.8-m (ERR 1-Jun-2019) × 3-mm; packed with 20% phase G1 on 80 - 100 mesh S1A support
4.2.2 Temperatures
Injection port: 225°
Detector: 250°
Column: 190°
Carrier gas: Nitrogen
Flow rate: 30 mL/min
Injection volume: 2 μL
4.2.3 System suitability
Sample: Standard solution
[Note - The relative retention times for p-chlorobenzaldehyde and 2,6-dichlorobenzaldehyde are 0.5 and 1.0, respectively.]
4.2.4 Suitability requirements
Resolution: NLT 3.0 between 2,6-dichlorobenzaldehyde and p-chlorobenzaldehyde
4.2.5 Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The relative peak response ratio from the Sample solution does not exceed that from the Standard solution (0.2%).
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 7 mg/mL
Acceptance criteria: 5.5–7.0
Loss on Drying 〈731〉
Analysis: Dry under vacuum at 60° for 2 h.
Acceptance criteria: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
USP Reference Standards 〈11〉
USP Guanabenz Acetate RS


