Granisetron Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
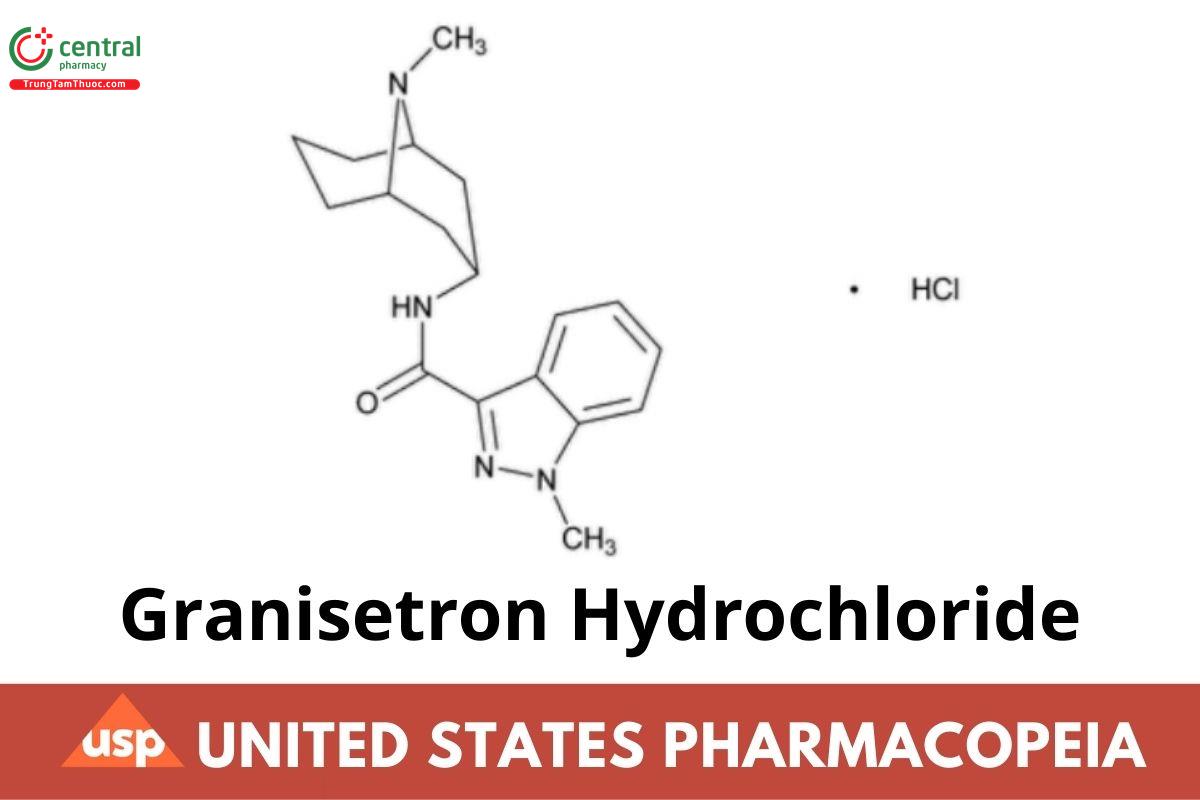
C18H24N4O · HCl 348.87
1H-Indazole-3-carboxamide, 1-methyl-N-(9-methyl-9-azabicyclo[3.3.1]-non-3-yl)-, monohydrochloride, endo-;
1-Methyl-N-(9-methyl-endo-9-azabicyclo[3.3.1]non-3-yl)-1H-indazole-3-carboxamide monohydrochloride CAS RN®: 107007-99-8; UNII: 318F6L70J8.
1 DEFINITION
Granisetron Hydrochloride contains NLT 97.0% and NMT 102.0% of granisetron hydrochloride (C18H24N4O · HCl), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride: Meets the requirements
3 ASSAY
Procedure
Mobile phase: Acetonitrile, water, phosphoric acid, and hexylamine (200: 800: 1.6: 1.0). Adjust with triethylamine to a pH of 7.5 ± 0.05 (about 4 mL is needed). Make adjustments if necessary. (See Chromatography 〈621〉, System Suitability.)
Standard solution: 1.0 mg/mL of USP Granisetron Hydrochloride RS in Mobile phase
System suitability solution: Transfer 2 mL of Standard solution to a colorless glass vial, stopper it, and either expose the solution to sunlight for 4 h or place it under a UV lamp for 16 h (granisetron undergoes partial degradation to granisetron related compound C). A degradation of at least about 0.3% of granisetron to granisetron related compound C must be obtained as shown by the appearance of a corresponding peak in the chromatogram. If it is not obtained, again expose the solution to sunlight or place it under a UV lamp. Sample solution: 1.0 mg/mL of Granisetron Hydrochloride in Mobile phase
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 305 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
3.2 System suitability
Samples: Standard solution and System suitability solution
Suitability requirements
Resolution: NLT 3.5 between granisetron related compound C and granisetron, System suitability solution
Tailing factor: NMT 2.0 for the granisetron peak, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of granisetron hydrochloride (C18H24N4O · HCl) in the portion of Granisetron Hydrochloride taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of granisetron from the Sample solution
rS = peak response of granisetron from the Standard solution
CS = concentration of USP Granisetron Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Granisetron Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
4.1 Organic Impurities
[Note—Protect all solutions containing Granisetron Hydrochloride from light.]
Mobile phase, System suitability solution, and Sample solution: Prepare as directed in the Assay.
Peak identification solution: 0.01 mg/mL of USP Granisetron Related Compound A RS and 0.005 mg/mL of USP Granisetron Related Compound B RS in Mobile phase
Standard solution: 0.005 mg/mL of USP Granisetron Hydrochloride RS in Mobile phase
Chromatographic system: Proceed as directed in the Assay, except for the Run time.
Run time: NLT 2 times the retention time of granisetron
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 3.5 between granisetron related compound C and granisetron, System suitability solution
Tailing factor: NMT 2.0 for the granisetron peak, System suitability solution
Relative standard deviation: NMT 10%, Standard solution
Analysis
Samples: Peak identification solution, Standard solution, and Sample solution
Calculate the percentage of each impurity in the portion of Granisetron Hydrochloride taken:
Result = (rU /rS ) × (CS /CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of granisetron from the Standard solution
CS = concentration of USP Granisetron Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Granisetron Hydrochloride in the Sample solution (mg/mL)
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1. Discard any peaks observed in the blank. The reporting threshold is 0.05%.
Table 1
Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
Granisetron related compound Da | 0.4 | 1.0 | 0.1 |
Granisetron related compound B | 0.5 | 0.59 | 0.5 |
Granisetron related compound A | 0.7 | 1.0 | 1.0 |
Granisetron related compound Cb | 0.8 | 1.0 | 0.2 |
Granisetron | 1.0 | − | − |
Any other individual impurity | − | 1.0 | 0.1 |
Total impurities | − | − | 1.0 |
a 1-Methyl -1H-indazole-3-carboxylic acid.
b N-[(1R,3r,5S)-9-Azabicyclo[3.3.1]non-3-yl]-1-methyl-1H-indazole-3-carboxamide.
4.2 Limit of Granisetron Related Compound E
Diluent: Acetonitrile and water (80:20)
Standard solution: 0.25 mg/mL of USP Granisetron Related Compound E RS in Diluent. [Note—USP Granisetron Related Compound E RS is the acetate salt of (1R,3r,5S)-9-Methyl-9-azabicyclo[3.3.1]nonan-3-amine. Use the correction factor stated on the label of the USP Reference Standard to calculate the concentration, as appropriate.]
Sample solution: 50 mg/mL of Granisetron Hydrochloride in Diluent
4.2.1 Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 2 µL
Developing solvent system: Ethyl acetate, isopropyl alcohol, and ammonium hydroxide (50: 30: 6.5)
4.2.2 Analysis
Samples: Standard solution and Sample solution
Proceed as directed in Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography and develop the chromatogram until the solvent front has moved about half of the length of the plate. Dry the plate in air, and expose it to iodine vapor for 30 min. Acceptance criteria: Any spot corresponding to granisetron related compound E from the Sample solution is not more intense than the corresponding spot from the Standard solution (0.5%).
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry at 105° for 4 h.
Acceptance criteria: NMT 0.5%
pH 〈791〉
Sample solution: 10 mg/mL in carbon dioxide-free water
Acceptance criteria: 4.0–6.5
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, protected from light. Store at room temperature.
USP Reference Standards 〈11〉
USP Granisetron Hydrochloride RS
USP Granisetron Related Compound A RS
2-Methyl-N-[(1R,3r,5S)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-2H-indazole-3-carboxamide.
USP Granisetron Related Compound B RS
N-[(1R,3r,5S)-9-Methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide.
USP Granisetron Related Compound E RS
(1R,3r,5S)-9-Methyl-9-azabicyclo[3.3.1]nonan-3-amine, acetate salt.


