Goserelin Implants
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Goserelin Implants are extended-release formulations of a sterile dispersion of Goserelin Acetate in a matrix of d,l-lactic and glycolic acids copolymer (PLGA) that is subcutaneously injected. The content of the PLGA and other ingredients is process-specific, and their identity and properties are determined by validated methods. The quality of Goserelin Acetate meets the compendial standard. Goserelin Implants contain NLT 90.0% and NMT 110.0% of the labeled amount of goserelin (C59H84N18O14).
2 IDENTIFICATION
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in Product-Related Substances and Impurities, Procedure 1.
3 ASSAY
Procedure
Buffer: 0.1 M sodium perchlorate solution, pH 2.1. [Note—This solution can be prepared as follows. Weigh 167.5 g of 60% (w/v) perchloric acid into a 1-L volumetric flask, then cool the flask on an ice bath. Add 1 M sodium hydroxide to volume. Transfer 100 mL of this 1 M sodium perchlorate solution to a 1-L volumetric flask and add water to volume. Adjust with 20% (w/v) sodium hydroxide to a pH of 2.1.] Mobile phase: Acetonitrile and Buffer (92:8)
Diluent: Acetonitrile and water (85:15)
System suitability solution: 2.0 mg/mL each of USP Goserelin Acetate RS and USP Goserelin Related Compound A RS in Diluent Standard solution: 2.0 mg/mL of USP Goserelin Acetate RS in Diluent
Sample solution: Nominally 2.0 mg/mL of goserelin in Diluent prepared by adding 10 Implants to a suitable volumetric flask. Add Diluent to about 70% of the total volume, and sonicate to dissolve the sample. Cool to room temperature, and dilute with Diluent to volume.
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 7.8-mm × 30-cm; 5-µm packing L33
Flow rate: 1.0 mL/min
Injection volume: 5 µL
Run time: 90 min
3.2 System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 4.0 between the main goserelin and goserelin related compound A peaks, System suitability solution Tailing factor: NMT 1.5 for the goserelin peak, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of goserelin (C59H84N18O14) in the portion of Implants taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of goserelin from the Sample solution
rS = peak response of goserelin from the Standard solution
CS = concentration of USP Goserelin Acetate RS in the Standard solution (mg/mL)
CU = nominal concentration of goserelin in the Sample solution (mg/mL)
Acceptance criteria: 90.0%–110.0%
4 PERFORMANCE TESTS
4.1 Dissolution 〈711〉
For 3.6-mg Implants
Medium: pH 7.4 phosphate/citrate buffer prepared as follows. Dissolve 25.8 g/L of anhydrous disodium hydrogen phosphate, 1.92 g/L of citric acid, and 0.2 g/L of sodium azide in water. Adjust with anhydrous disodium hydrogen phosphate or citric acid to a pH of 7.4. Pass through a sterile filter of NMT 0.2-µm pore size into a sterile container; 50 mL
Apparatus: 120-mL at-bottomed, borosilicate glass jar with a tight plastic cap. Incubate at 39 ± 0.5°. [Note—Suitable procedures should be carried out to remove/minimize microbiological contamination on the glassware immediately before the test, e.g., rinse with methanol and dry at 90° for 2 h.]
Times: 168, 336, 408, 504, and 672 h
Sample solutions: Place 5 Implants in each jar, and add 50 mL of Medium at ambient temperature to each jar. Place the jars in the incubator. At the times specified, remove the jars from the incubator and allow to cool for 1 h. Swirl gently to mix, and withdraw 5 mL of aliquot. Replace the aliquot withdrawn with the same volume of Medium at ambient temperature into the jar, and return to the incubator 2 h after removal. Dilute each sample with Medium (1:1).
For 10.8-mg Implants
Medium: pH 7.4 phosphate buffered saline prepared as follows. Dissolve 8 g/L of sodium chloride, 0.19 g/L of potassium dihydrogen phosphate, 1.38 g/L of anhydrous disodium hydrogen phosphate, and 0.2 g/L of sodium azide in water. Adjust with 2 M hydrochloric acid to a pH of 7.4. Pass through a sterile filter of NMT 0.2-µm pore size into a sterile container; 50 mL.
Apparatus: 120-mL at-bottomed, borosilicate glass jar with a tight plastic cap. Incubate at 39 ± 0.5°. [Note—Suitable procedures should be carried out to remove/minimize microbiological contamination on the glassware immediately before the test, e.g., rinse with methanol and dry at 90° for 2 h.]
Times: 72, 336, 840, 1344, and 2016 h
Sample solutions: Transfer 50 mL of Medium to the jars, seal, and warm to 39 ± 0.5° overnight in the incubator. Place a single Implant in each jar, and return to the incubator. At the times specified, remove the jars from the incubator, swirl gently to mix, and withdraw 5 mL of aliquot at 24, 72, 168, and 264 h, and 20 mL of aliquot at 336, 504, 672, 840, 1008, 1176, 1344, 1512, 1680, 1848, and 2016 h. Replace the aliquot withdrawn with the same volume of prewarmed Medium into the jar, and return to the incubator. [Note—At each sampling time point, the UV absorbance must be measured so that the goserelin content of the test aliquot can be used in the calculation of cumulative release.]
Standard solution: 0.1 mg/mL of USP Goserelin Acetate RS in Medium
Analytical wavelength: 280 nm
[Note—Bandwidth is 10 nm.]
Path length cell: 1 cm
Blank: Medium
Analysis
Samples: Sample solutions and Standard solution
Calculate each Sample solution concentration of goserelin taken at each sampling time point, Cx :
Result = (AU /AS ) × CS
AU = absorbance of each Sample solution taken at each sampling time point
AS = absorbance of the Standard solution
CS= concentration of USP Goserelin Acetate RS in the Standard solution (mg/mL)
Calculate the cumulative amount of goserelin (C59H84N18O14) dissolved, as a percentage of the labeled amount of the dose, at the specified times:
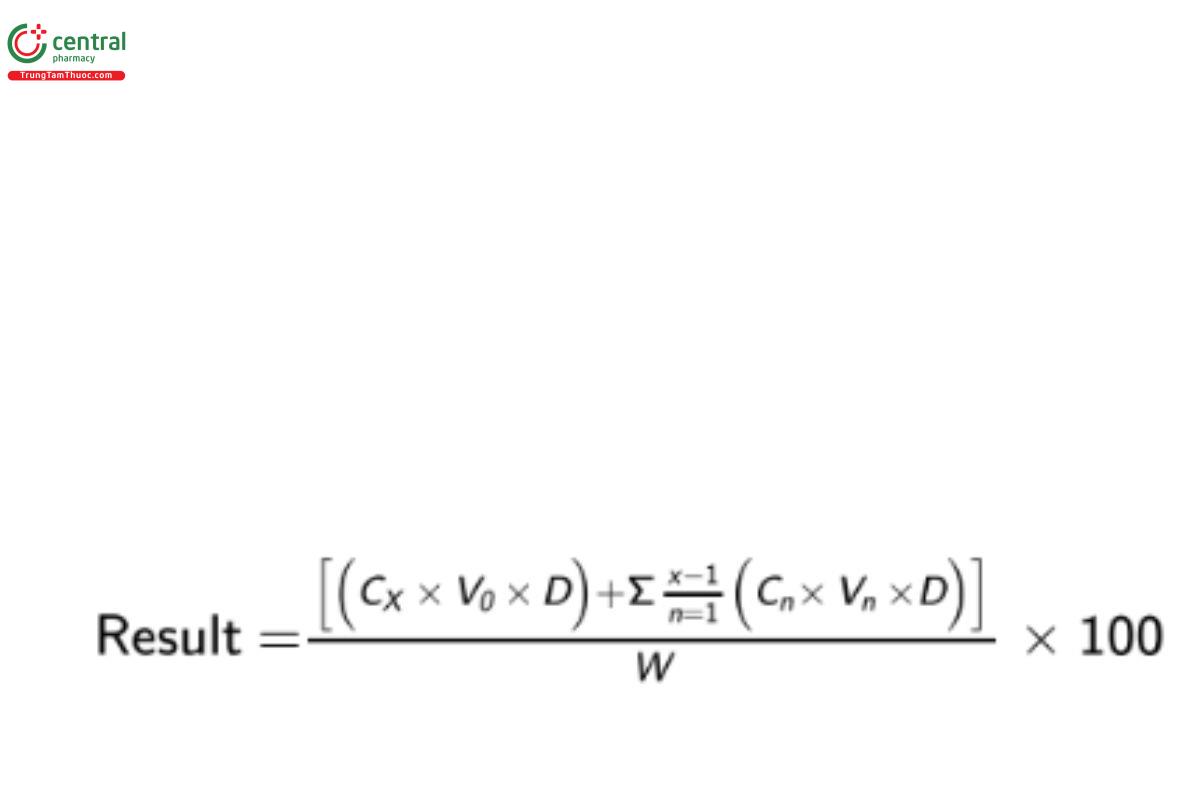
CX = concentration of goserelin in each Sample solution taken at each sampling time point (mg/mL)
V0 = volume of the dissolution Medium, 50 mL
D = dilution factor
Cn = concentration of goserelin in each Sample solution taken at n sampling time point (mg/mL), n must be ≤ x−1
Vn = volume of each Sample solution taken at n sampling time point (mL)
W = amount of goserelin in the sample (mg)
Tolerances: See Table 1.
Table 1
Time (h) | For 3.6-mg Implants Only Amount Dissolved (%) | For 10.8-mg Implants Only Amount Dissolved (%) | |
Each of the Three Replicates (Individually) | Mean of the 6 Implants | Individual Implant | |
72 | — | 10–25 | 5–30 |
168 | NMT 20 | — | — |
336 | 25–55 | 15–40 | 10–45 |
408 | 35–75 | — | — |
504 | 65–90 C | — | — |
672 | 85–105 | — | — |
840 | — | 20–55 | 15–60 |
1344 | — | 60–85 | 55–90 |
2016 | — | NLT 75 | NLT 70 |
4.2 Uniformity of Dosage Units 〈905〉
Mobile phase: 2.72 mg/mL of potassium dihydrogen phosphate in a mixture of water and methanol (45:55). Adjust with phosphoric acid to a pH of 3.0.
Diluent: Acetonitrile and water (85:15)
Standard solution: 0.2 mg/mL of USP Goserelin Acetate RS in Diluent
Sample solution: Nominally 0.2 mg/mL of goserelin in Diluent prepared by dissolving 1 Implant in a suitable volume of Diluent with the aid of sonication
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 35°
Flow rate: 1.8 mL/min
Injection volume: 10 µL
Run time: 12 min
4.2.2 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5 for the goserelin peak
Relative standard deviation: NMT 1.5%
4.2.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of goserelin (C59H84N18O14) in the portion of Implant taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of goserelin from the Sample solution
rS = peak response of goserelin from the Standard solution
CS = concentration of USP Goserelin Acetate RS in the Standard solution (mg/mL)
CU = nominal concentration of goserelin in the Sample solution (mg/mL)
Acceptance criteria: Meet the requirements
5 PRODUCT-RELATED SUBSTANCES AND IMPURITIES
5.1 Procedure 1
Mobile phase: 2.72 mg/mL of potassium dihydrogen phosphate in a mixture of acetonitrile and water (27:73). Adjust with phosphoric acid to a pH of 3.0.
Diluent: Acetonitrile and water (85:15)
System suitability solution: 20 µg/mL each of USP Goserelin Acetate RS and USP Goserelin Related Compound A RS in Diluent Sensitivity solution: 3 µg/mL of USP Goserelin Acetate RS in Diluent
Standard solution: 20 µg/mL of USP Goserelin Acetate RS in Diluent
Sample solution: Nominally 2.0 mg/mL of goserelin prepared by dissolving 10 Implants in Diluent with the aid of sonication
5.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1.8 mL/min
Injection volume: 5 µL
Run time: 60 min
5.1.2 System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[Note—The retention times for goserelin related compound A and goserelin in the System suitability solution are approximately 17 and 22 min, respectively.]
Suitability requirements
Resolution: NLT 4.5 between the main goserelin and goserelin related compound A peaks, System suitability solution Tailing factor: NMT 2.0 for the goserelin peak, Standard solution
Relative standard deviation: NMT 4.5%, Standard solution
Signal-to-noise ratio: NLT 5, Sensitivity solution
5.1.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Implants taken:
Result = (rU /rS ) × (CS /CU) × F
rU = peak response of any individual impurity from the Sample solution
rS = peak response of goserelin from the Standard solution
CS = concentration of USP Goserelin Acetate RS in the Standard solution (µg/mL)
CU = nominal concentration of goserelin in the Sample solution (mg/mL)
F = conversion factor, 0.1
Acceptance criteria
Any individual impurity: NMT 1.0%
Total impurities: NMT 4.0% from Procedure 1
5.2 Procedure 2
Buffer, Mobile phase, Diluent, System suitability solution, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Sensitivity solution: 6.5 µg/mL of USP Goserelin Acetate RS in Diluent
Peak identification solution: Degrade Implants at 90° for 4 h. Dissolve the degraded Implants in Diluent with the aid of sonication to obtain a solution that contains approximately 2 mg/mL of goserelin.
5.2.1 System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 4.0 between the main goserelin and goserelin related compound A peaks, System suitability solution Tailing factor: NMT 1.5 for the goserelin peak, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 5, Sensitivity solution
5.2.2 Analysis
Samples: Standard solution, Sample solution, and Peak identification solution
Identify the O-glycolyl-ser4-goserelin and polymer envelope peaks using the chromatogram of the Peak identification solution and the relative retention times listed in Table 2.
Calculate the percentage of any individual impurity in the portion of Implants taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of any individual impurity from the Sample solution
rS = peak response of goserelin from the Standard solution
CS = concentration of USP Goserelin Acetate RS in the Standard solution (mg/mL)
CU = nominal concentration of goserelin in the Sample solution (mg/mL)
Acceptance criteria: See Table 2.
Table 2
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
Polymer envelopea | 0.10–0.60 | 5.5 |
O-Glycolyl-ser4-goserelin | 0.93 | 2.0 |
Any unspecified impurity | — | 1.0 |
Total impuritiesb | — | 10.0 |
a O-Lactide/glycolide copolymer chain to ser4 of goserelin. It is calculated as the sum of all peaks eluted in the specified relative retention time range.
b Total impurities is the sum of impurities from Procedure 2 and impurity C from Procedure 3.
5.3 Procedure 3
[Note—Perform this test if impurity C is present.]
Buffer: Prepare as directed in the Assay.
Mobile phase: Acetonitrile and Buffer (87.5:12.5)
Diluent: Acetonitrile and water (85:15)
System suitability solution: Weigh 4 mg of USP Goserelin Acetate RS, add 250 µL of triuoroacetic acid, and leave to stand for 24 h. Dissolve in 20 mL of Diluent to obtain a solution containing goserelin related compound B. [Note—Goserelin related compound B is des-t-butyl goserelin.]
Sensitivity solution: 3 µg/mL of USP Goserelin Acetate RS in Diluent
Standard solution: 20 µg/mL of USP Goserelin Acetate RS in Diluent
Sample solution: Nominally 2.0 mg/mL of goserelin in Diluent prepared by adding 10 Implants to a suitable volumetric flask. Add Diluent to about 70% of the total volume, and sonicate to dissolve the sample. Cool to room temperature, and dilute with Diluent to volume.
5.3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 7.8-mm × 30-cm; 5-µm packing L33
Flow rate: 2.0 mL/min
Injection volume: 50 µL
Run time: 30 min
5.3.2 System suitability
Samples: System suitability solution and Sensitivity solution
Suitability requirements
Retention: The retention time for goserelin related compound B is between 19 and 24 min, System suitability solution Signal-to-noise ratio: NLT 5, Sensitivity solution
5.3.3 Analysis
Samples: System suitability solution, Standard solution, and Sample solution
Identify the impurity C peak with a retention time relative to goserelin related compound B of 1.1 using the chromatogram of the System suitability solution. [Note—Impurity C is a specified, but unidentified, impurity.]
Calculate the percentage of impurity C in the portion of Implants taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of impurity C from the Sample solution
rS = peak response of goserelin from the Standard solution
CS = concentration of USP Goserelin Acetate RS in the Standard solution (mg/mL)
CU = nominal concentration of goserelin in the Sample solution (mg/mL)
Acceptance criteria: NMT 1.0%
6 OTHER COMPONENTS
Acetic Acid Content
Standard stock solution: 6.25 mg/mL of USP Glacial Acetic Acid RS in dimethylformamide
Internal standard stock solution: Transfer 1.0 mL of n-hexadecane to a 50-mL volumetric flask containing approximately 30 mL of dimethylformamide. Dilute with dimethylformamide to volume.
Standard solution: Transfer 10.0 mL of the Standard stock solution to a 100-mL volumetric flask, and add approximately 50 mL of dimethylformamide. Add 5.0 mL of the Internal standard stock solution, and dilute with dimethylformamide to volume.
Sample solution: Weigh 150 mg of Implants into a 5-mL volumetric flask. Add approximately 1 mL of dimethylformamide and sonicate to dissolve. Add 250 µL of the Internal standard stock solution, and dilute with dimethylformamide to volume.
6.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 10-m; fused-silica capillary that contains a 0.3-µm lm of phase G35
Temperatures
Injection port: 200°
Column: See Table 3.
Table 3
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
50 | 0 | 50 | 0.1 |
50 | 30 | 200 | 3 |
Detector: 250°
Carrier gas: Helium
Flow rate: 1.3 mL/min
Injection volume: 1 µL
Split ratio: 100:30
6.2 System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 15 between acetic acid and n-hexadecane
Tailing factor: NMT 2.0 for acetic acid
Relative standard deviation: NMT 6% for the response ratio of acetic acid to n-hexadecane
6.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of acetic acid in the portion of Implants taken:
Result = (RU /RS ) × (CS /CU) × 100
RU = peak response ratio of acetic acid to n-hexadecane from the Sample solution
RS = peak response ratio of acetic acid to n-hexadecane from the Standard solution
CS = concentration of USP Glacial Acetic Acid RS in the Standard solution (mg/mL)
CU = nominal concentration of the goserelin implant sample in the Sample solution (mg/mL)
Acceptance criteria: NMT 2.5%
7 SPECIFIC TESTS
Water Determination 〈921〉, Method I, Method Ic
Sample solution: 27-mg/mL solution of Implant in dry dimethylformamide
Analysis: Determine the water content of 1.0-mL of the Sample solution. Perform a blank determination and make any necessary correction. Acceptance criteria: NMT 2.5%
Bacterial Endotoxins Test 〈85〉: NMT 350 USP Endotoxin Units/Implant
Sterility Tests 〈71〉, Test for Sterility of the Product to be Examined, Membrane Filtration: Meet the requirements
8 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, tight, light-resistant single-unit containers at controlled room temperature.
Labeling: The label states the amount of the peptide (goserelin) in mg in each implant.
USP Reference Standards 〈11〉
USP Glacial Acetic Acid RS
USP Endotoxin RS
USP Goserelin Acetate RS
USP Goserelin Related Compound A RS
4-d-Serine goserelin.


