Goserelin Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
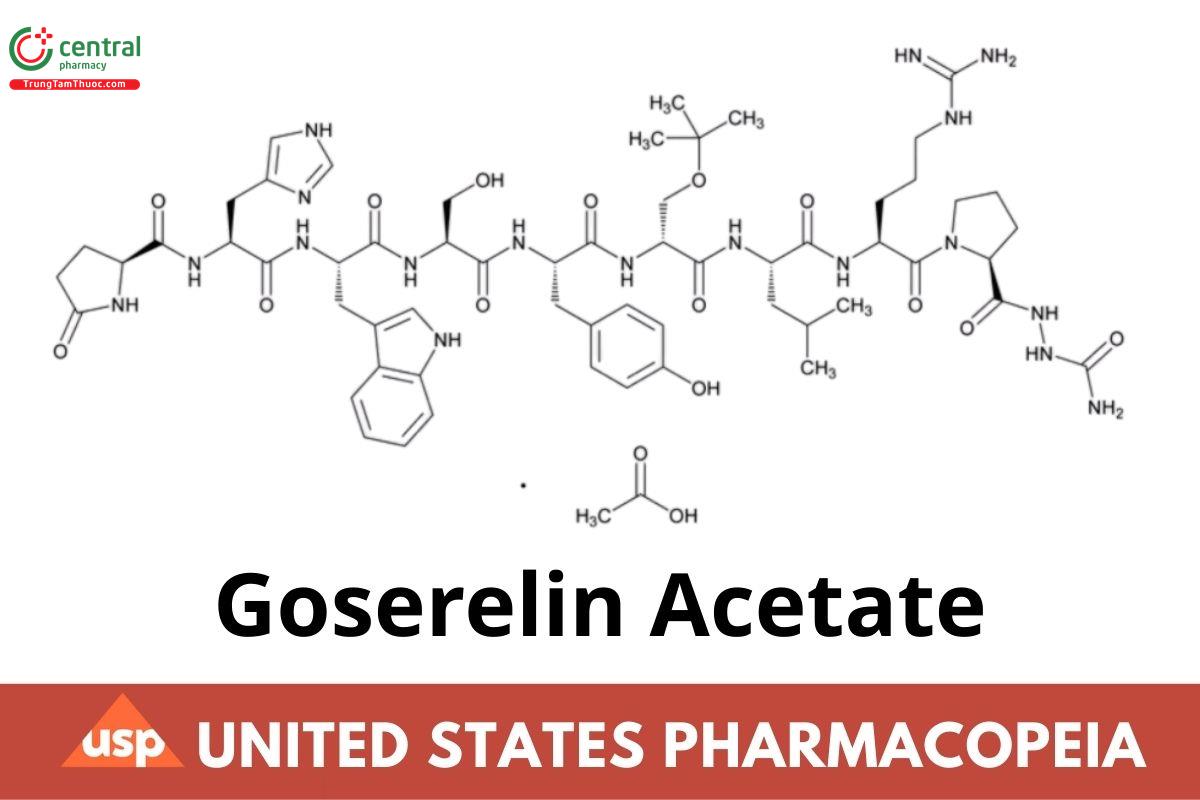
C59H84N18O14 · xC2H4O2 1269.43 (as free base)
Luteinizing hormone-releasing factor (pig), 6-[O-(1,1-Dimethylethyl)-d-serine]-10-deglycinamide-, 2-(aminocarbonyl)hydrazide, acetate (salt); 1-(5-Oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-O-tert-butyl-d-seryl-l-leucyl-l-arginyl-l-prolyl)semicarbazide (goserelin free base); Free base: CAS RN®: 65807-02-5; UNII: 0F65R8P09N.
Acetate salt: CAS RN®: 145781-92-6; UNII: 6YUU2PV0U8.
1 DEFINITION
Goserelin Acetate is a synthetic nonapeptide analog of the hypothalmic decapeptide, Gonadorelin. It is obtained by chemical synthesis and is available as an acetate salt. It contains NLT 94.5% and NMT 103.0% of goserelin (C59H84N18O14), calculated on the anhydrous and acetic acid free basis.
2 IDENTIFICATION
A. The retention time of the goserelin peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: Prepare a filtered and degassed mixture of water, acetonitrile, and trifluoroacetic acid (1600:400:1). Standard solution: 1 mg/mL of USP Goserelin Acetate RS in water
Diluted standard solution: Transfer 1 mL of the Standard solution to a 10-mL volumetric ask, and dilute with water to volume. System suitability solution 1: Prepare a solution of 0.1 mg/mL USP Goserelin Related Compound A RS in water, and mix with an equal volume of Diluted standard solution.
System suitability solution 2: Prepare USP Goserelin System Suitability Mixture RS as indicated on the label.
Sample solution: 1 mg/mL of Goserelin Acetate in water
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Column temperature: 50°–55°
Flow rate: 1 mL/min
Injection volume: 10 µL
3.2 System suitability
Samples: Standard solution, System suitability solution 1, and System suitability solution 2
[Note—For System suitability solution 1, the retention time for the goserelin peak is between 40 and 50 min; see Table 1 for the relative retention times. Two minor peaks are visible prior to the elution of the principal peak, System suitability solution 2.]
Suitability requirements
Resolution: NLT 7.0 between the goserelin and goserelin related compound A (4-d-Ser-goserelin) peaks, System suitability solution 1 Relative standard deviation: NMT 2.0% for the goserelin peak from replicate injections, Standard solution
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of goserelin (C59H84N18O14) in the portion of Goserelin Acetate taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 94.5%–103.0% on the anhydrous, acetic acid-free basis
4 OTHER COMPONENTS
Acetic Acid in Peptides 〈503〉: 4.5%–15.0%
5 IMPURITIES
Change to read:
Organic Impurities
Related Compounds
Mobile phase, Standard solution, Diluted standard solution, System suitability solution 1, System suitability solution 2, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Diluted sample solution: Transfer 1 mL of the Sample solution into a 100-mL volumetric ask, and dilute with water to volume.
5.1 System suitability
Samples: System suitability solution 1 and System suitability solution 2
[Note—For System suitability solution 1, the retention time for the goserelin peak is between 40 and 50 min; see Table 1 for the relative retention times. For System suitability solution 2, two peaks, corresponding to decarbamoylgoserelin and 2-d-His-goserelin and eluting prior to the principal peak, are visible.]
Resolution: NLT 7.0, System suitability solution 1
Column efficiency: NLT 2000 theoretical plates, System suitability solution 1
Tailing factor: NMT 2.0, System suitability solution 1
Relative standard deviation: NMT 2.0%, System suitability solution 2
Table 1
Name | Relative Retention Time |
4-d-Ser-goserelin | 0.67 |
Decarbamoylgoserelin | 0.89 |
5-d-Tyr-goserelin | 0.92 |
2-d-His-goserelin | 0.94 |
Goserelin (ERR 1-Dec-2020) | 1.0 |
5.2 Analysis
Samples: Sample solution and Diluted sample solution
Calculate the percentage of goserelin-related impurities in the portion of Goserelin Acetate taken:
Result = rI /rU
rI = peak response for any individual impurity in the Sample solution
rU = peak response of the main goserelin peak in the Diluted sample solution
Acceptance criteria
Decarbamoylgoserelin: NMT 1.0%
Any other impurity: NMT 0.5%
Total impurities: NMT 2.5%
6 SPECIFIC TESTS
Amino acid Content
(See Nuclear Magnetic Resonance Spectroscopy 〈761〉.)
[Note—Concentrations of goserelin in the Standard solution and the Sample solution must be the same (within 5% of each other) but can be adjusted based on the quality of the carbon-13 spectra obtained. The spectra must be acquired under the same conditions for both the Standard solution and the Sample solution. The spectra obtained are of sufficient quality to allow quantification of the integrals of the resonances specified in this test. Integrals and spectra of the Standard solution and the Sample solution can be repeated and averaged.]
Standard solution: Dissolve USP Goserelin Acetate RS in deuterium oxide to obtain a solution having a known concentration of about 10% (w/v), and adjust with deuterated acetic acid-d4 to a pH of 4.
Sample solution: Prepare a 10% (w/v) solution of Goserelin Acetate in deuterium oxide, and adjust with deuterated acetic acid-d4 to a pH of 4.
Analysis
Samples: Standard solution and Sample solution
Obtain a carbon-13, proton-decoupled nuclear magnetic resonance (NMR) spectrum of both the Standard solution and the Sample solution. The spectra from the solutions are qualitatively similar, and all the resonances from the spectrum of the Standard solution are present in the spectrum of the Sample solution and have the same chemical shift values (±0.1 ppm for goserelin, ±0.5 ppm for acetate). Identify any other resonances in the spectrum of the Sample solution. Integrate the resonances at the approximate parts per million corresponding to each amino acid in Table 2.
Table 2
Amino Acids | Resonances (ppm) |
Azo-glycine | 162.2 |
Histidine | 118.4 |
Tyrosine | 116.7 |
tert-Butyl serine | 62.2 |
Serine | 62.5 |
Tryptophan | 55.7 |
Arginine | 41.8 |
Pyroglutamic acid | 26.3 |
Proline | 26.0 |
Leucine | 23.5 |
Calculate the ratio of each of the amino acids from the integrals of the Standard solution and the Sample solution:
Result = rU /rS
rU = integral of the resonance of a designated amino acid from the Sample solution
rS = integral of the resonance of a designated amino acid from the Standard solution
Acceptance criteria: 0.9–1.1 for histidine, tyrosine, tert-butyl serine, serine, tryptophan, Arginine, pyroglutamic acid, proline, and leucine ; 0.8– 1.2 for azo-glycine
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 2 mg/mL, in water, calculated on the anhydrous and acetic acid-free basis
Acceptance criteria: Between −52° and −56°
Bacterial Endotoxins Test 〈85〉: The level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Goserelin Acetate is used can be met. Where the label states Goserelin Acetate must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Goserelin Acetate is used can be met.
Water Determination 〈921〉, Method I: NMT 10.0%
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers, and store in a refrigerator.
USP Reference Standards 〈11〉
USP Goserelin Acetate RS
USP Goserelin Related Compound A RS
USP Goserelin System Suitability Mixture RS


