Glycine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
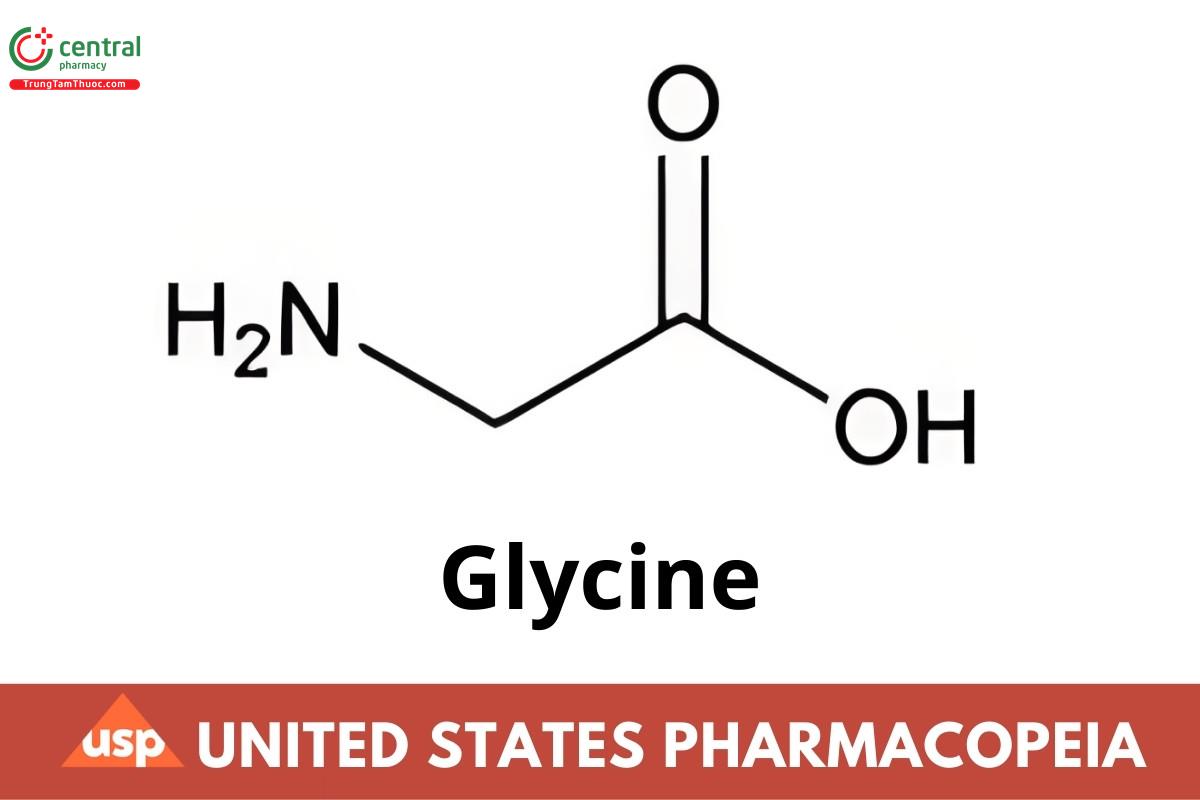
C2H5NO2 75.07
Glycine CAS RN®: 56-40-6.
1 DEFINITION
Glycine contains NLT 98.5% and NMT 101.5% of glycine (C2H5NO2), calculated on the dried basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M
3 ASSAY
PROCEDURE
Sample: 150 mg of Glycine
Blank: 100 mL of glacial acetic acid
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 100 mL of glacial acetic acid, and add 1 drop of crystal violet TS. Titrate with the Titrant to a green endpoint.
Perform the Blank determination.
Calculate the percentage of glycine (C2H5NO2) in the Sample taken:
Result = {[(VS - VB) x N x F]/W} x 100
VS = Titrant volume consumed by the Sample (mL)
VB = Titrant volume consumed by the Blank (mL)
N = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 75.07 mg/mEq
W = Sample weight (mg)
Acceptance criteria: 98.5%-101.5% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%
4.2 CHLORIDE AND SULFATE (221), Chloride
Standard solution: 0.10 mL of 0.020 N hydrochloric acid
Sample: 1 g of Glycine
Acceptance criteria: NMT 0.007%
4.3 CHLORIDE AND SULFATE (221), Sulfate
Standard solution: 0.20 mL of 0.020 N sulfuric acid
Sample: 3 g of Glycine
Acceptance criteria: NMT 0.0065%
4.4 HYDROLYZABLE SUBSTANCES
Sample solution: 100 mg/mL of Glycine
Analysis: Boil 10 mL of the Sample solution for 1 min, and set aside for 2 h.
Acceptance criteria: The solution appears as clear and as mobile as 10 mL of the same solution that has not been boiled.
Change to read:
4.5 RELATED COMPOUNDS
Solution A: Transfer 2.16 g of octanesulfonic acid sodium salt to a 1000-mL volumetric flask, add 900 mL of HPLC grade water and 2.0 mL of perchloric acid, and mix to dissolve. Adjust with 5 N sodium hydroxide solution to a pH of 2.2. Dilute with HPLC grade water to volume. Pass the solution through a membrane filter of 0.2-µm pore size.
Solution B: Acetonitrile
Mobile phase: Gradient elution. See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 7 | 100 | 0 |
| 13 | 90 | 10 |
| 18 | 90 | 10 |
| 35 | 100 | 0 |
| 45 | 100 | 0 |
Standard solution: A mixture of 0.005 mg/mL each of USP Glycine RS, USP Diglycine RS, USP Triglycine RS, and glycine anhydride,1 and 0.0025 mg/mL of monochloroacetic acid2 in HPLC grade water. [NOTE-Monochloroacetic acid may be omitted from the Standard solution if the article being tested does not contain this substance.]
Sample solution: Transfer 125 mg of Glycine into a 25-mL volumetric flask, dissolve in and dilute with HPLC grade water to volume.
Blank: HPLC grade water
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 4.6-mm × 15-cm; 3-µm packing L1
Column temperature: 25°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Samples: Standard solution and Blank
[NOTE-See Table 2 for the relative retention times.]
Suitability requirements
Interference peaks: Compare the chromatogram obtained from the Standard solution with that obtained from the Blank. Any peak area from the Blank that overlaps or co-elutes with the Amino acid peak from the Standard solution is NMT 2.0% of that amino acid peak area.
Resolution: NLT 2.0 between the diglycine and triglycine peaks, Standard solution
Relative standard deviation: NMT 5.0% each for the specified peaks, Standard solution
Analysis
Samples: Standard solution, Sample solution, and Blank
Separately inject the Blank, Standard solution, and Sample solution into the chromatograph. Compare the chromatogram from the Sample solution with that from the Blank. Disregard any peak observed in both the Sample solution and the Blank. Identify the amino acid impurities in the Sample solution by comparing with those specified in the Standard solution.
Separately calculate the percentage of each specified impurity in the portion of Glycine taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of glycine anhydride, monochloroacetic acid, diglycine, or triglycine from the Sample solution
rS = peak response of glycine anhydride, monochloroacetic acid, diglycine, or triglycine from the Standard solution
CS = concentration of glycine anhydride, monochloroacetic acid, USP Diglycine RS, or USP Triglycine RS in the Standard solution (mg/mL)
CU = concentration of Glycine in the Sample solution (mg/mL)
Separately calculate the percentage of iminodiacetic acid, hexamethylenetetramine, and any unspecified impurity in the portion of Glycine taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of iminodiacetic acid, hexamethylenetetramine, or any unspecified impurity from the Sample solution
rS = peak response of glycine from the Standard solution
CS = concentration of USP Glycine RS in the Standard solution (mg/mL)
CU = concentration of Glycine in the Sample solution (mg/mL)
Acceptance criteria: See Table 2. [NOTE-Disregard any impurity peak less than 0.05%.]
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Glycine anhydride | 0.25 | 0.1 |
| Monochloroacetic acid | 0.44 | — (RB 1-Nov-2021) |
| Iminodiacetic acid | 0.60 | 0.1 |
| Glycine | 1.00 | — |
| Diglycine | 1.70 | 0.1 |
| Triglycine | 1.80 | 0.1 |
| Hexamethylenetetramine | 2.47 | 0.1 |
| Any unspecied impurity | — | — (RB 1-Nov-2021) |
| Total impurities | — | 1.0 |
5 SPECIFIC TESTS
LOSS ON DRYING (731)
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in well-closed containers at room temperature.
6.2 USP REFERENCE STANDARDS (11)
USP Diglycine RS
USP Glycine RS
USP Triglycine RS
1 Analytical grade with purity NLT 99.0%.
2 Analytical grade with purity NLT 99.0%.


