Glutaral Concentrate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
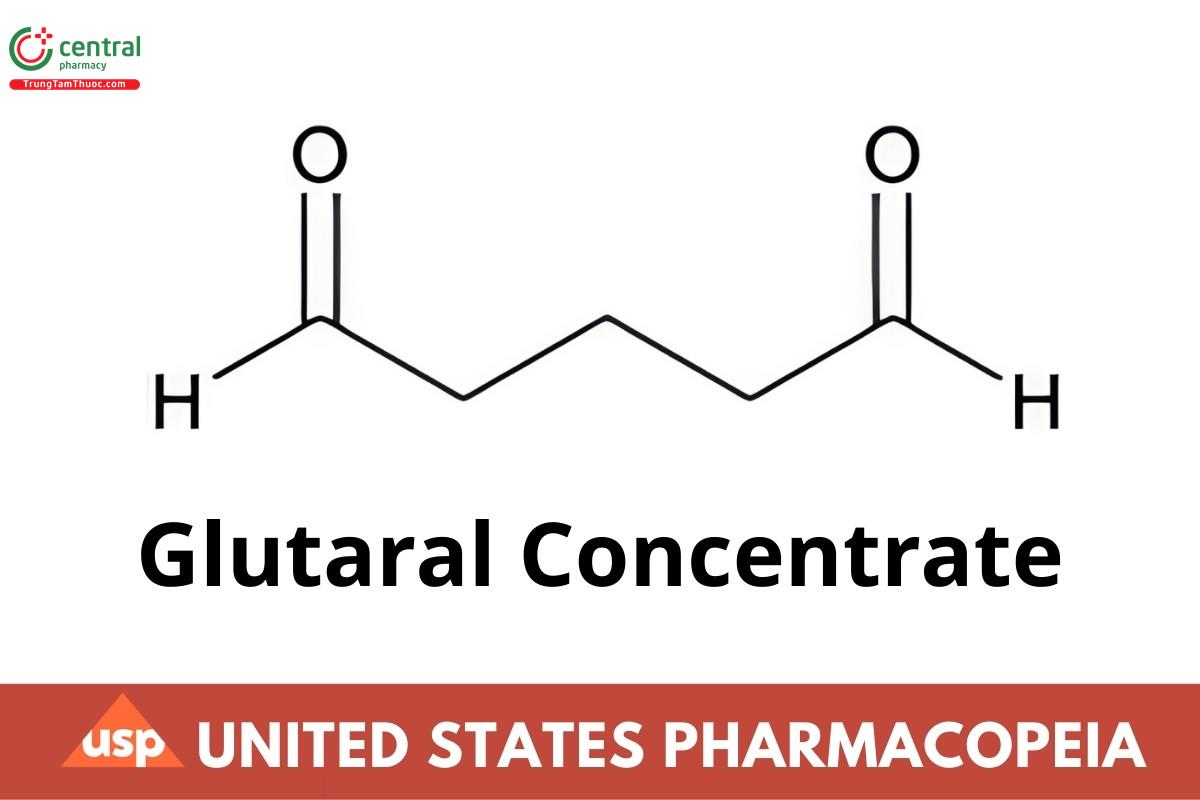
C5H8O2 100.12
Pentanedial;
Glutaraldehyde CAS RN®: 111-30-8; UNII: T3C89M417N.
1 DEFINITION
Glutaral Concentrate is a solution of glutaraldehyde in Purified Water. It contains NLT 100.0% and NMT 104.0% of the labeled amount of glutaral (C5H8O2). The labeled amount is 50.0 g of C5H8O2 per 100 g of Concentrate.
2 IDENTIFICATION
A.
Solution A: Add 4 mL of sulfuric acid to 0.8 g of 2,4-dinitrophenylhydrazine, then add 6 mL of water, dropwise, with swirling. When dissolution is essentially complete, add 20 mL of alcohol, and filter. The filtrate is the 2,4-dinitrophenylhydrazine reagent.
Analysis: Add 0.4 mL of Concentrate to 20 mL of Solution A, mix by swirling, and allow to stand for 5 min. Collect the precipitate on a filter, and rinse thoroughly with alcohol. Dissolve the precipitate in 20 mL of hot ethylene dichloride, filter, and cool the filtrate in an ice bath until crystallization occurs. Collect the precipitate on a filter. Redissolve the precipitate by refluxing with 30 mL of acetone, filter, and cool the filtrate in an ice bath until crystallization occurs. Collect the precipitate on a filter.
Acceptance criteria: The 2,4-dinitrophenylhydrazone so obtained melts at 185°-195°, within a 3° range (see Melting Range or Temperature (741)).
3 ASSAY
Change to read:
PROCEDURE
Solution A: 35 g/L of hydroxylamine hydrochloride prepared as follows. Dissolve 35 g of hydroxylamine hydrochloride in 150 mL of water in a 1000-mL volumetric flask. Add isopropyl alcohol to volume.
Solution B: Transfer 65 mL of triethanolamine to a glass-stoppered, 1000-mL volumetric flask, and add water to volume.
Analysis: Το 500 mL of Solution A add 15 mL of a solution of bromophenol blue in alcohol (1 in 2500), and add Solution B from a buret to obtain a neutralized solution that appears greenish-blue by transmitted light. Transfer 65.0 mL of the neutralized solution to a glass-stoppered, 500-mL conical flask, add 50.0 mL of Solution B, purge with nitrogen, and insert the stopper. Add 1.2 g of Glutaral Concentrate. Insert the stopper, and allow to stand for 60 min, swirling the flask occasionally. Titrate with 0.5 N sulfuric acid VS to a greenish-blue endpoint, and perform a blank determination (see Titrimetry (541), Residual Titrations).
Calculate the percentage of the labeled amount of glutaral (C5H8O2) in the Concentrate taken:
Result = {[(VB - VS) x N x F]/W) x 100
VB = volume of 0.5 N sulfuric acid VS consumed by the blank (mL)
VS = volume of 0.5 N sulfuric acid VS consumed by the Sample solution (mL)
N = normality of the sulfuric acid
F = equivalency factor for glutaraldehyde, 0.05006 g/mEq
W = nominal weight of glutaral (ERR 1-Dec-2018) taken (g)
Acceptance criteria: 100.0%-104.0%
IMPURITIES
4 SPECIFIC TESTS
4.1 SPECIFIC GRAVITY (841)
1.126-1.135 at 20°
4.2 ACIDITY
Analysis: Transfer 60.0 g to a conical flask, add phenolphthalein TS, and titrate with 0.10 N alcoholic potassium hydroxide to a pink endpoint that is permanent for NLT 15 s.
Acceptance criteria: NMT 40 mL of 0.10 N potassium hydroxide is consumed, corresponding to NMT 0.4% (w/w) of acid, calculated as acetic acid.
4.3 PH (791)
3.7-4.5
4.4 CLARITY OF SOLUTION
Analysis: Transfer 5.0 mL of Concentrate to a glass-stoppered, 100-mL graduated cylinder, add water to obtain 100 mL of mixture, insert the stopper, and mix by inverting the graduated cylinder several times. Allow the bubbles to rise, and view downward through the solution against a dark background.
Acceptance criteria: The solution is clear.
5 ADDITIONAL REQUIREMENTS
5.1 PACKAGING AND STORAGE
Preserve in tight containers, protected from light, and avoid exposure to excessive heat.
5.2 LABELING
The label states that this article is not intended for direct administration to humans or animals.


