Gentamicin Sulfate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
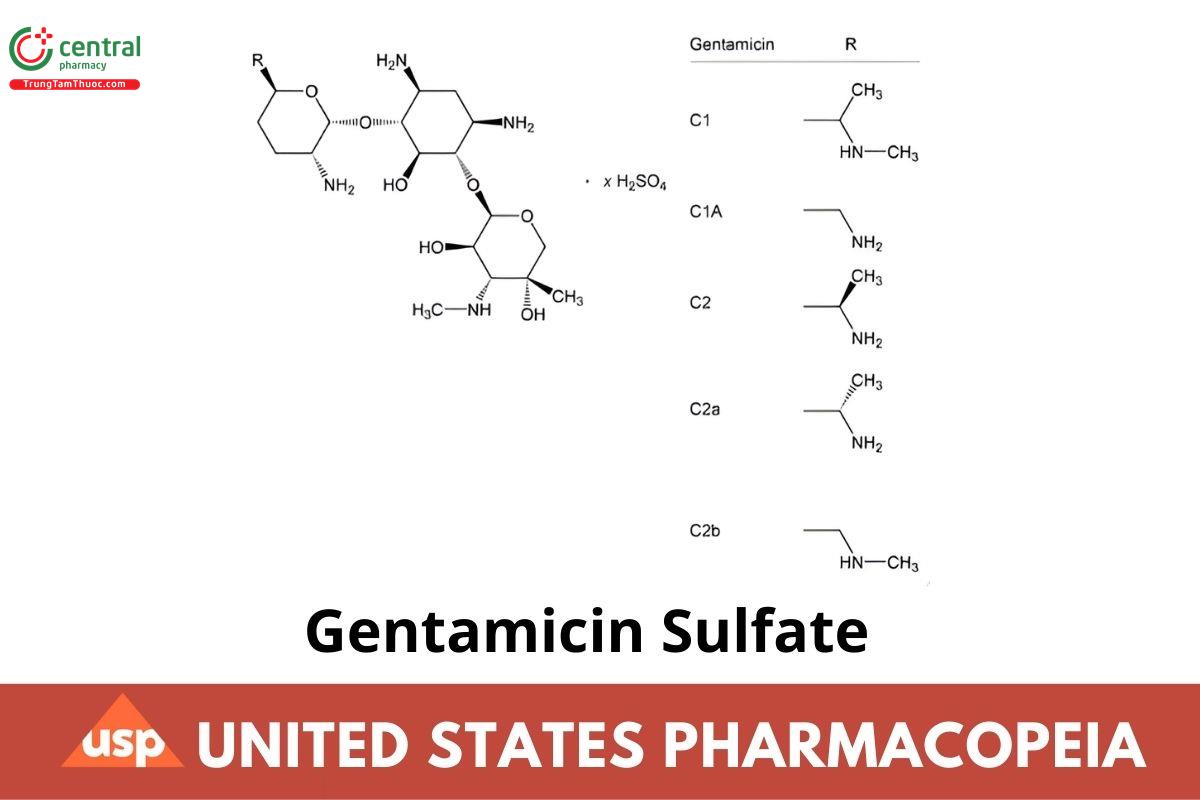
Gentamicin sulfate (salt);
Gentamycin sulfate
CAS RN®: 1405-41-0; UNII: 8X7386QRLV.
1 DEFINITION
Gentamicin Sulfate is the sulfate salt, or a mixture of such salts, of the antibiotic substances produced by the growth of Micromonospora purpurea. It has a potency equivalent to NLT 590 μg/mg of gentamicin, calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. Identification Tests - General, Sulfate 〈191〉
3 ASSAY
Procedure
Analysis: Proceed as directed for Gentamicin under Antibiotics—Microbial Assays 〈81〉.
Acceptance criteria: NLT 590 μg/mg of gentamicin, calculated on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 1.0%
Limit of Methanol (if present)
Internal standard solution: 0.50% v/v of n-propyl alcohol
Standard solution: 0.25% v/v each of methanol and n-propyl alcohol
Control solution: 250 mg/mL of Gentamicin Sulfate
Sample solution: 250 mg/mL of Gentamicin Sulfate in a mixture of Internal standard solution and water (1:1)
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 4-mm × 1.5-m; packed with support S3
Temperature
Column: Constant temperature between 120° and 140°
Injector: Constant temperature at least 50° higher than the column temperature
Detector: Constant temperature at least 50° higher than the column temperature
Carrier gas: Nitrogen
Flow rate: Constant flow rate between 30 and 40 mL/min
Injection type: Syringe with a polytef-tipped plunger
Injection volume: 2 μL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 1.0 between n-propyl alcohol and methanol
Analysis
Samples: Standard solution, Sample solution, and Control solution
Chromatograph the Control solution, and examine the chromatogram: if any peak is observed at a retention time corresponding to that of n-propyl alcohol, use the response of that peak to correct the n-propyl alcohol peak response of the Sample solution.
Calculate the percentage of methanol in the Gentamicin Sulfate taken:
Result = (RU/RS) × (CS/CU) × D × F
RU = peak area response of methanol to n-propyl alcohol (corrected for any peak at the locus of the n-propyl alcohol peak in the Control solution) from the Sample solution
RS = peak area response of methanol to n-propyl alcohol from the Standard solution
CS = percentage of methanol in the Standard solution
CU = concentration of Gentamicin Sulfate in the Sample solution (mg/mL)
D = density of methanol (g/mL)
F = conversion factor, 1000 mg/g
Acceptance criteria: NMT 1.0%
5 SPECIFIC TESTS
5.1 Content of Gentamicins
Mobile phase: To 900 mL of carbonate-free water, add 7 mL of trifluoroacetic acid, 250 μL of pentafluoropropanoic acid, and 4 mL of 12.5 M sodium hydroxide (carbonate-free). Allow to equilibrate, and adjust with 0.5 M sodium hydroxide (carbonate-free) to a pH of 2.6. Add 15 mL of acetonitrile, and dilute with carbonate-free water to 1 L. If necessary, adjust the volume of acetonitrile in the Mobile phase. A total volume of up to 50 mL can be added per L of Mobile phase.
System suitability solution: 100 μg/mL of USP Gentamicin Sulfate RS and 20 μg/mL of USP Sisomicin Sulfate RS in Mobile phase
Sample solution: 0.2 mg/mL of Gentamicin Sulfate in Mobile phase
5.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Pulsed amperometric electrochemical detector
Indicator electrode: Gold
Reference electrode: Silver–silver chloride
Auxiliary electrode: Stainless steel. [Note—If the cell body is made of stainless steel, it can be used as the auxiliary electrode.]
Waveform: See Table 1.
Table 1
| Time (s) | Potential (V) | Integration |
|---|---|---|
| 0.00 | +0.05 | – |
| 0.10 | +0.05 | Begin |
| 0.40 | +0.05 | End |
| 0.41 | +0.75 | – |
| 0.55 | +0.75 | – |
| 0.56 | −0.15 | – |
| 1.00 | −0.15 | – |
Column: 4.6-mm × 25-cm; 5-μm packing L1
Column temperature: 35°
Flow rate: 1 mL/min
Post-column reagent: 20 g/L sodium hydroxide (carbonate-free), degassed and introduced pulselessly using a 375-μL polymeric mixing coil. [Note-A suitable mixing coil is the knitted reaction coil, part number 043700, available from Dionex Corporation (www.dionex.com).]
Flow rate of post-column reagent: 0.3 mL/min
Injection volume: 20 μL
Run time: 1.2 times the retention time of gentamicin C
5.1.2 System suitability
Sample: System suitability solution
5.1.3 Suitability requirements
Resolution: NLT 1.5 between gentamicin C and gentamicin C
5.1.4 Analysis
Sample: Sample solution
Calculate the percentage of each gentamicin in the portion of Gentamicin Sulfate taken:
Result = (rU/rT) × 100
rU = peak area response corresponding to the particular gentamicin
rT = sum of the area responses of the gentamicin C1a, gentamicin C2, gentamicin C2a, gentamicin C2b, and gentamicin C1 peaks
Acceptance criteria: Identify peaks by the relative retention times in Table 2.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Garaminea,b | 0.35 | – |
| Sisomicina,c | 1.0 | – |
| Gentamicin C1a | 1.1 | 10%–35% |
| Gentamicin C2 | 1.8 | 25%–55%d |
| Gentamicin C2a | 2.3 | |
| Gentamicin C2b | 2.0 | 25%–50%e |
| Gentamicin C1 | 2.9 |
a These compounds are listed for information only and are not to be reported in this test.
b 4-O-[3-Deoxy-4-C-methyl-3-(methylamino)-β-l-arabinopyranosyl]-2-deoxy-l-streptamine.
c O-3-Deoxy-4-C-methyl-3-(methylamino)-β-l-arabinopyranosyl-(1→4)-O-[2,6-diamino-2,3,4,6-tetradeoxy-α-d-glycero-hex-4-enopyranosyl-(1→6)]-2-deoxy-d-streptamine.
d The limit is for the sum of gentamicin C and gentamicin C .
e The limit is for the sum of gentamicin C and gentamicin C .
5.2 Optical Rotation, Specific Rotation
Sample solution: 10 mg/mL
Acceptance criteria: +107° to +121°
pH 〈791〉
Sample solution: 40 mg/mL
Acceptance criteria: 3.5–5.5
5.3 Loss on Drying
Analysis: Dry it in vacuum at a pressure NMT 5 mm of mercury at 110° for 3 h.
Acceptance criteria: NMT 18.0%
Sterility Tests 〈71〉: Where the label states that Gentamicin Sulfate is sterile, it meets the requirements.
Bacterial Endotoxins Test 〈85〉: Where the label states that Gentamicin Sulfate is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.71 USP Endotoxin Unit/mg of gentamicin.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Gentamicin Sulfate RS
USP Sisomicin Sulfate RS


