Gelatin
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Portions of this monograph that are national USP text, and are not part of the harmonized text, are marked with symbols to specify this fact.
1 DEFINITION
Purified protein obtained from Collagen of animals (including fish and poultry), by partial alkaline and/or acid hydrolysis, and/or enzymatic hydrolysis, or by thermal hydrolysis.
The hydrolysis leads to gelling or non-gelling grades. This monograph covers both the gelling grades and non-gelling grades (called also hydrolyzed gelatin).
2 IDENTIFICATION
2.1 A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A
[NOTE-Compare the spectra of the test specimen and USP Gelatin RS over the range from 800 to 3500 cm-1. Disregard any peak at about 1403 cm-1.]
2.2 B.
Sample solution: Dissolve 1.00 g in carbon dioxide-free water at about 55°, dilute with the same solvent to 100 mL, and keep at 55°. Save the unused portion of this solution for use in the test for pH.
Analysis: To 2 mL of the Sample solution add 0.05 mL of a 125-g/L solution of copper sulfate pentahydrate. Mix, and add 0.5 mL of an 85-g/L solution of sodium hydroxide.
Acceptance criteria: A violet color is produced.
2.3 C.
Sample: 0.5 g
Analysis: Place the Sample in a test tube of about 15-mm internal diameter, and add 10 mL of water. Allow to stand for 10 min, heat at 60° for 15 min, and keep the tube upright at 2°- 8° for 6 h. Invert the tube.
Acceptance criteria: The contents do not flow out immediately for gelling grades. The contents immediately flow out for non-gelling grades.
2.4 D. FOR NON-GELLING GRADES
Medium: pH 6.8 phosphate buffer. Mix 77.3 mL of a 71.5-g/L solution of disodium hydrogen phosphate dodecahydrate with 22.7 mL of a 21-g/L solution of citric acid.
Sample: 0.5 g
Color reagent: Prepare immediately before use. Dissolve 1.0 g of dimethylaminobenzaldehyde in 3.5 mL of perchloric acid [600 g/L of perchloric acid (HCIO)].
Analysis: Place the Sample in a 250-mL bottle. Add 10 mL of water and 5 mL of sulfuric acid. Place the bottle, partly but not completely closed (for example, using a watch glass), in an oven at 105° for 4 h. Allow to cool, and add 200 mL of water. Adjust with a 200-g/L solution of sodium hydroxide to a pH of 6.0-8.0. Place 2 mL of the solution in a test tube, and add 2 mL of oxidizing reagent (14-g/L solution of chloramine T in the Medium; prepare immediately before use). Mix, and allow to stand for 20 min. Add 2 mL of Color reagent, and slowly add 6.5 mL of 2-propanol). Mix, and place in a water bath at 60° for about 15 min.
Acceptance criteria: A red to violet color develops.
3 SPECIFIC TESTS
3.1 PH (791)
Sample: Use the Sample solution prepared in Identification B.
Acceptance criteria: 3.8-7.6 at 55°
3.2 WATER CONDUCTIVITY (645)
Sample: 1.0% solution at 30 ± 1.0°
Analysis: Determine without the use of the temperature compensation.
Acceptance criteria: NMT 1 mS cm-1
Change to read:
3.3 SULFUR DIOXIDE
Sample: 25.0 g
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N sodium hydroxide VS
Endpoint detection: Visual
Analysis: Introduce 150 mL of water into the flask (see A in Figure 1), and pass carbon dioxide through the whole system for 15 min at a rate of 100 mL/min. To 10 mL of hydrogen peroxide solution [30 g/L of hydrogen peroxide (H₂O₂)] add 0.15 mL of bromophenol blue TS. Add
▲Titrant ▲(ERR 1-Nov-2020) until a violet-blue color is obtained, without exceeding the endpoint. Place the solution in the test tube (D). Without interrupting the stream of carbon dioxide, remove the funnel (B), and introduce through the opening into the flask (A) the Sample with the aid of 100 mL of water. Add through the funnel 80 mL of diluted hydrochloric acid [73 g/L of hydrogen chloride (HCI)], and boil for 1 h. Open the tap of the funnel, stop the flow of carbon dioxide, and also stop the heating and the cooling water. Transfer the contents of the test tube with the aid of a little water to a 200-mL wide-necked, conical flask. Heat on a water bath for 15 min, and allow to cool. Add 0.1 mL of bromophenol blue TS, and titrate with Titrant until the color changes from yellow to violet-blue.
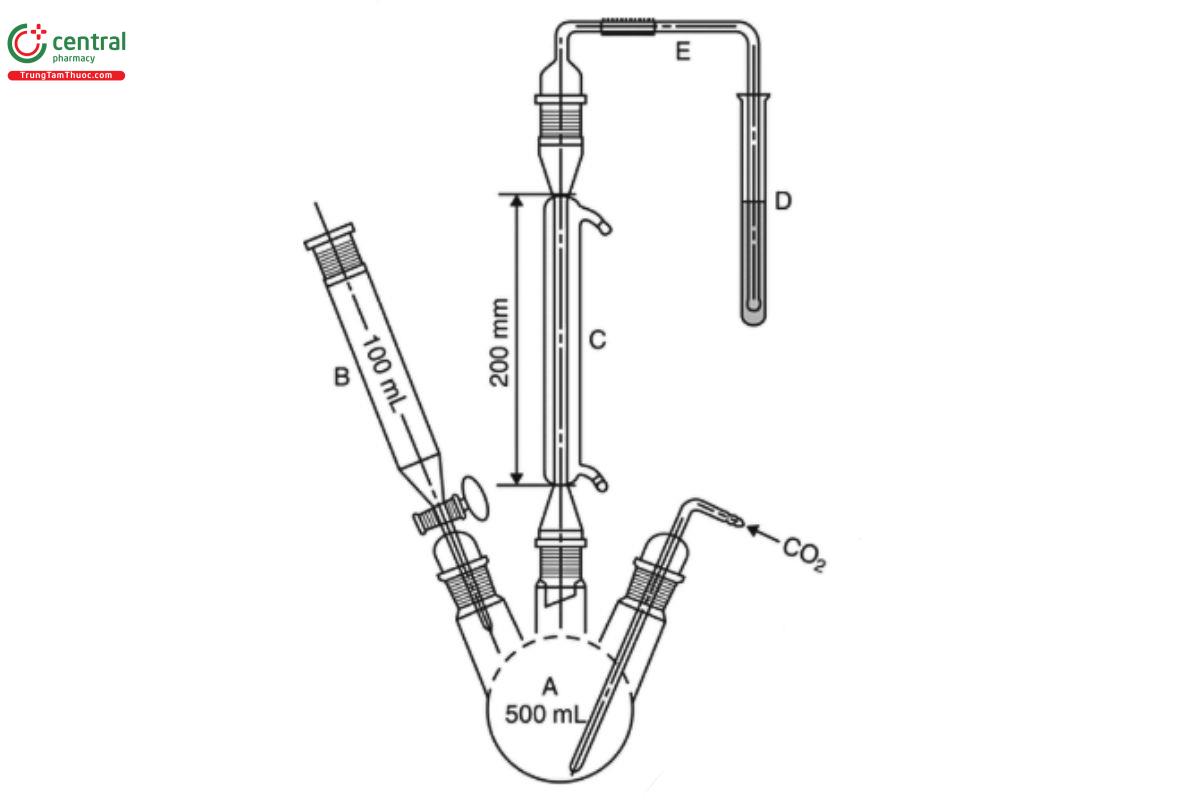
Carry out a blank titration, and calculate the content of sulfur dioxide, in ppm:
Result = [(VS - VB) x (▲N▲(ERR 1-Nov-2020)/W)] × 32,030
VS = volume of ▲Titrant ▲(ERR 1-Nov-2020) consumed by the Sample (mL)
VB = volume of ▲Titrant ▲(ERR 1-Nov-2020) consumed by the blank (mL)
▲NA ▲(ERR 1-Nov-2020) = actual ▲normality ▲(ERR 1-Nov-2020) of the Titrant ▲▲(ERR 1-Nov-2020)
W = weight of the Sample (g)
Acceptance criteria: NMT 50 ppm
3.4 PEROXIDES
Peroxidase transfers oxygen from peroxides to an organic redox indicator that is converted to a blue oxidation product. The intensity of the color obtained is proportional to the quantity of peroxide and can be compared with a color scale provided with the test strips to determine the peroxide concentration.
Peroxide test strips: Use commercial test strips with a suitable scale covering the range of 0-25 ppm of peroxide.
Sample: 20.0 ± 0.1 g
Suitability test
Hydrogen peroxide standard solution: 2 ppm of hydrogen peroxide (H2O2) prepared by dilution of hydrogen peroxide solution [30 g/L of hydrogen peroxide (H2O2)]
Procedure: Dip a test strip for 1 s into Hydrogen peroxide standard solution, such that the reaction zone is properly wetted. Remove the test strip, shake off excess liquid, and after 15 s, compare the reaction zone with the color scale provided. The test strips are suitable if the color matches that of the 2-ppm concentration.
Analysis: Weigh the Sample in a beaker, and add 80.0 ± 0.2 mL of water. Stir to moisten all of the gelatin, and allow the Sample to stand at room temperature for 1-3 h. Cover the beaker with a watch glass. If the Sample is not dissolved completely, place the beaker in a water bath at 65 ± 2° for 20 ± 5 min to dissolve the Sample. Stir the contents of the beaker with a glass rod to achieve a homogeneous solution. Dip a test strip for 1 s into the test solution, such that the reaction zone is properly wetted. Remove the test strip, shake off excess liquid, and after 15 s, compare the reaction zone with the color scale provided. Multiply the concentration read from the color scale by a factor of 5 to calculate the concentration, in ppm, of peroxide in the test substance.
Acceptance criteria: NMT 10 ppm
3.5 GEL STRENGTH (BLOOM VALUE)
For gelling grades
The gel strength is expressed as the mass in grams necessary to produce the force that, applied to a plunger 12.7 mm in diameter, makes a depression 4-mm deep in a gel with a concentration of 6.67% m/m and matured at 10°.
Sample: 7.5 g
Apparatus: Texture analyzer or gelometer with a cylindrical piston 12.7 ± 0.1 mm in diameter with a plane pressure surface and a sharp bottom edge, and a bottle 59 ± 1 mm in internal diameter and 85 mm high. Adjust the apparatus according to the manufacturer's manual.
Settings
Distance: 4 mm
Test speed: 0.5 mm/s
Analysis: Place the Sample in a bottle. Add 105 mL of water, place a watch glass over the bottle, and allow to stand for 1-4 h. Heat in a water bath at 65 ± 2° for 15 min. While heating, stir gently with a glass rod. Ensure that the solution is uniform and that any condensed water on the inner walls of the bottle is incorporated. Allow to cool at room temperature for 15 min, and transfer the bottle to a thermostatically controlled bath at 10.0 ± 0.1°, fitted with a device to ensure that the platform on which the bottle stands is perfectly horizontal. Close the bottle with a rubber stopper, and allow to stand for 17 ± 1 h. Remove the Sample bottle from the bath, and quickly wipe the water from the exterior of the bottle. Center the bottle on the platform of the Apparatus so that the plunger contacts the Sample as nearly at its midpoint as possible, and start the measurement.
Acceptance criteria: 80%-120% of the labeled nominal value
3.6 IRON
3.6.1 Atomic absorption spectrometry, standard additions method
Iron standard solution (8 ppm): Dissolve 80 mg of iron in 50 mL of hydrochloric acid [220 g/L of hydrogen chloride (HCI)], and dilute with water to 1000.0 mL. Immediately before use, prepare a 1:10 dilution with water.
Standard solution: Prepare using the Iron standard solution, diluted with water as necessary.
Sample solution: In a conical flask, add 10 mL of hydrochloric acid [37% m/m of hydrogen chloride (HCI)] to 5.00 g of the substance to be examined. Close the flask, and place in a water bath at 75°-80° for 2 h. (If necessary for proper solubilization, the gelatin may be allowed to swell after addition of the acid and before heating, the heating time may be prolonged, and a higher temperature may be used.) Allow to cool, and adjust the contents of the flask with water to 100.0 g.
3.6.2 Instrumental conditions
(See Atomic Absorption Spectroscopy (852).)
Analytical wavelength: 248.3 nm
Acceptance criteria: NMT 30 ppm
3.7 CHROMIUM
3.7.1 Atomic absorption spectrometry, standard additions method
Chromium standard solution (100 ppm): 0.283 mg/mL of potassium dichromate (K2Cr2O7) in water
Standard solution: Prepare using the Chromium standard solution, diluted with water if necessary.
Sample solution: Use the Sample solution described in the test for Iron.
3.7.2 Instrumental conditions
(See Atomic Absorption Spectroscopy (852).)
Analytical wavelength: 357.9 nm
Acceptance criteria: NMT 10 ppm
3.8 ZINC
3.8.1 Atomic absorption spectrometry, standard additions method
Zinc standard solution (10 ppm): Dissolve 0.440 g of zinc sulfate heptahydrate and 1 mL of acetic acid [300 g/L of acetic acid (C2H4O2)] in water, and dilute to 100.0 mL. Immediately before use, prepare a 1:100 dilution in water.
Standard solution: Prepare using the Zinc standard solution, diluted with water if necessary.
Sample solution: Use the Sample solution described in the test for Iron.
3.8.2 Instrumental conditions
(See Atomic Absorption Spectroscopy (852).)
Analytical wavelength: 213.9 nm
Acceptance criteria: NMT 30 ppm
3.9 LOSS ON DRYING (731)
Sample: 5.0 g
Analysis: Dry the Sample in an oven at 105° for 16 h.
Acceptance criteria: NMT 15%
3.10 MICROBIAL ENUMERATION TESTS (61), and TESTS FOR SPECIFIED MICROORGANISMS (62)
The total bacterial count does not exceed 103 cfu/g, the total yeasts and molds count does not exceed 102 cfu/g, and the tests for Salmonella species and Escherichia coli are negative.
4 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Protect from heat and moisture.
LABELING: The label states the Gel Strength (Bloom Value) or that it is a non-gelling grade.
USP Reference Standards 〈11〉
USP Gelatin RS


