Gadoterate Meglumine Injection
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
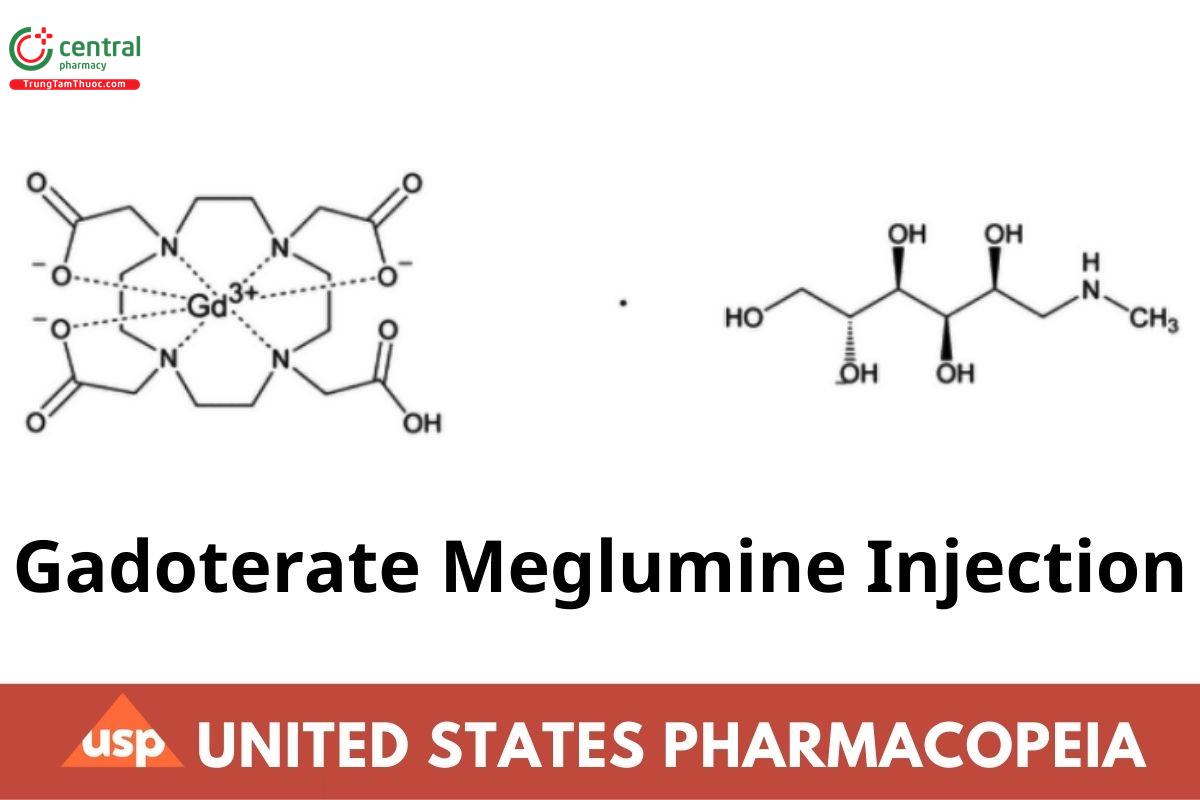
C16H25GdN4O8 · C7H17NO5 753.86
d-Glucitol, 1-deoxy-1-(methylamino)-, [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetato(4-)-
κN1,κN4,κN7,κN10,κO1,κO4,κO7,κO10]gadolinate(1-) (1:1);
1-Deoxy-1-(methylamino)-d-glucitol hydrogen [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetato(4-)-
κN1,κN4,κN7,κN10,κO1,κO4,κO7,κO10]gadolinate(1-) CAS RN®: 92943-93-6.
1 DEFINITION
Gadoterate Meglumine Injection is a sterile solution of Gadoterate Meglumine in Water for Injection. It contains NLT 90.0% and NMT 110.0% of the labeled amount of gadoterate meglumine (C16H25GdN4O8 · C7H17NO5 ).
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Analytical wavelength: 265–285 nm
Sample solution: Dilute Injection with water (1:10).
Acceptance criteria: The absorption spectrum exhibits maxima at 273 and 276 nm.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Buffer 1: 5.5 g/L of ammonium acetate. Adjust with acetic acid to a pH of 6.0.
Buffer 2: 2.1 g/L of ammonium acetate. Adjust with acetic acid to a pH of 6.0.
Mobile phase: Acetonitrile and Buffer 1 (65:35)
Diluent: Acetonitrile and Buffer 2 (60:40)
Standard solution: 3.6 mg/mL of USP Gadoterate Meglumine RS in Diluent. Vigorous shaking may be required for complete dissolution. Sample solution: Nominally 3.6 mg/mL of gadoterate meglumine from a suitable volume of Injection in Diluent. Vigorous shaking may be required for complete dissolution.
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Fluorescence
Excitation wavelength: 274 nm
Emission wavelength: 312 nm
Column: 4.6-mm × 25.0-cm; 5-µm packing L114
Column temperature: 50°
Flow rate: 0.8 mL/min
Injection volume: 5 µL
Run time: NLT 2.5 times the retention time of gadoterate
3.2 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2
Relative standard deviation: NMT 1.0%
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of gadoterate meglumine (C16H25GdN4O8 · C7H17NO5) in the portion of Injection taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of fluorescence emission for gadoterate from the Sample solution
rS = peak response of fluorescence emission for gadoterate from the Standard solution
CS = concentration of USP Gadoterate Meglumine RS in the Standard solution (mg/mL)
CU = nominal concentration of gadoterate meglumine in the Sample solution (mg/mL)
Acceptance criteria: 90.0%–110.0%
4 OTHER COMPONENTS
Change to read:
Content of Meglumine
Sample solution: Gadoterate Meglumine Injection
Blank: Water
Instrumental conditions
Mode: Polarimetry (see Optical Rotation 〈781S〉, Procedures, Specic Rotation), except for Temperature
Temperature: 20°
Lamp: Sodium
Analytical wavelength: 589 nm
Cell: 1 dm
Analysis:
Samples: Sample solution and Blank
Determine the angular rotation (see Optical Rotation 〈781〉).
Calculate the amount of meglumine as a percentage of the formulation content of meglumine (C7H17NO5) in the portion of Injection 7 17 5
taken:
Result = (a/α) × 100 (ERR 1-Jun-2021) × (1/l) × (1/L) × 100
a = observed angular rotation of the Sample solution corrected for the Blank (°)
α = average specific rotation of meglumine, 24.0 deg · dm−1 · g−1 · dL
l = cell path length, dm
L = formulation content of meglumine in the Injection, 9.76 g/dL
Acceptance criteria: 90.0%–110.0%
5 IMPURITIES
5.1 Limit of Free Gadolinium
Buffer: 5.7 mL/L of glacial acetic acid and 8.2 g/L of sodium acetate (10:20). [Note—If necessary, adjust with 5.7 mL/L of glacial acetic acid or 8.2 g/L of sodium acetate to a pH between 4.8 and 5.2.]
Indicator solution: 0.1% xylenol orange in water
Sample solution: Transfer 20 mL of Injection into a suitable ask. Add 30 mL of water, 10 mL of Buffer, 1 drop of pyridine, and 5–10 drops of Indicator solution.
Blank: Water
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.01 M edetate disodium VS
Endpoint detection: Visual
Analysis
Sample: Sample solution
Titrate with Titrant to a yellow endpoint. Add an additional drop of pyridine. If a violet color appears, continue the analysis until it turns yellow.
Calculate the percentage of free gadolinium (% w/v) in the portion of Injection taken:
Result = [VT × M × F × (1/VU)] × (1/10)
VT = volume of Titrant consumed (mL)
M = molarity of Titrant (mM/mL)
F = equivalency factor, 157.25 mg/mM
VU = volume of Injection used to prepare the Sample solution, 20 mL
Acceptance criteria: NMT 0.001% (w/v)
5.2 Limit of Free Tetraxetan
Buffer 1: 5.7 mL/L of glacial acetic acid and 8.2 g/L of sodium acetate (10:20). [Note—If necessary, adjust with 5.7 mL/L of glacial acetic acid or 8.2 g/L of sodium acetate to a pH between 4.8 and 5.2.]
Buffer 2: 0.5 g/L of copper sulfate in water
Sample solution: Transfer 5 mL of Injection into a suitable ask. Add 5 mL each of Buffer 1 and Buffer 2.
Blank: Transfer 5 mL of water into a suitable ask. Add 5 mL each of Buffer 1 and Buffer 2.
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.002 M edetate disodium VS
Endpoint detection: Potentiometric. [Note—An electrode system consisting of a copper indicator electrode and a calomel reference electrode is recommended.]
Analysis
Samples: Sample solution and Blank
Titrate potentiometrically with Titrant.
Calculate the percentage of free tetraxetan (% w/v) in the portion of Injection taken:
Result = [(VB − VT ) × M × F] × (1/VU ) × (1/10)
VB = volume of Titrant consumed by the Blank (mL)
VT = volume of Titrant consumed by the Sample solution (mL)
M = actual molarity of Titrant (mM/mL)
F = equivalency factor, 404.42 mg/mM
VU = volume of Injection used to prepare the Sample solution, 5 mL
Acceptance criteria: 0.005%–0.05% (w/v)
6 SPECIFIC TESTS
pH 〈791〉: 6.5–8.0
Particulate Matter in Injections 〈788〉: It meets the requirements for small-volume injections.
Sterility Tests 〈71〉: Meets the requirements
Bacterial Endotoxins Test 〈85〉: Meets the requirements
Container Content for Injections 〈697〉: Meets the requirements
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at controlled room temperature.
USP Reference Standards 〈11〉
USP Gadoterate Meglumine RS.


