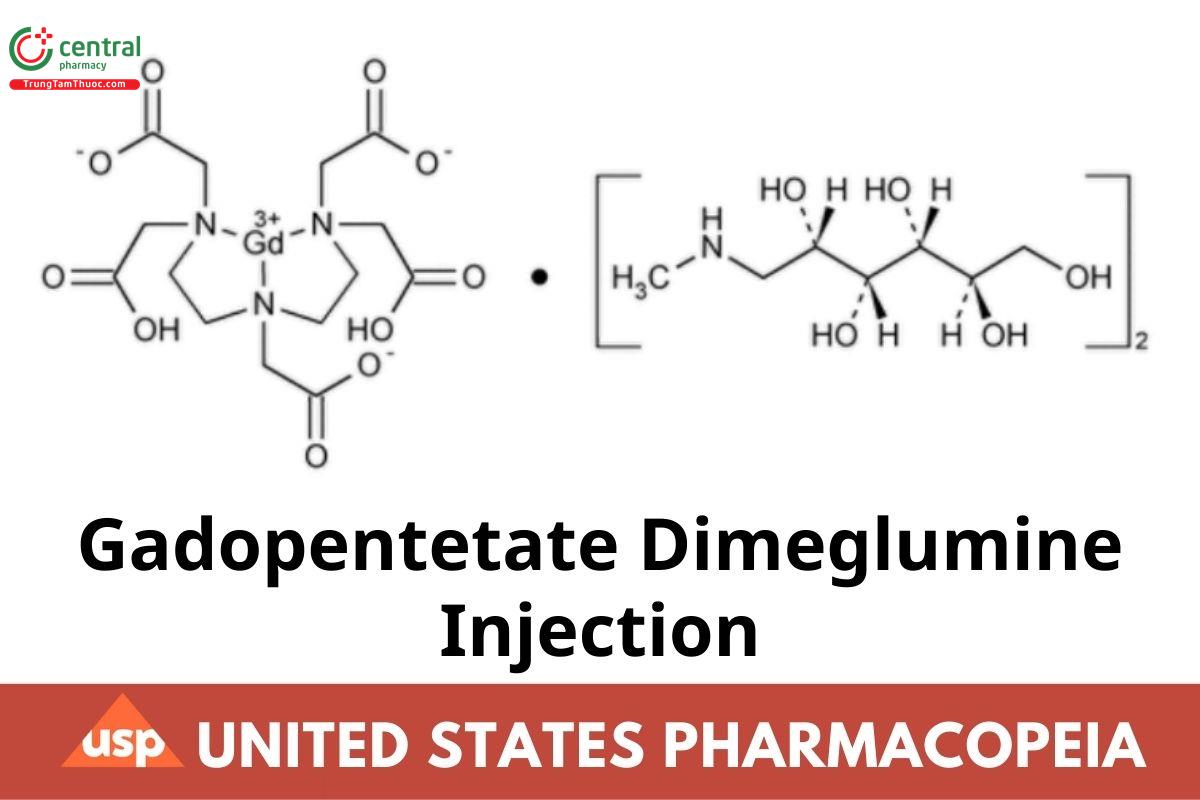
Gadopentetate Dimeglumine Injection
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Gadopentetate Dimeglumine Injection is a sterile solution of gadopentetate dimeglumine in Water for Injection. It contains NLT 90.0% and NMT 110.0% of the labeled amount of gadopentetate dimeglumine (C14H20GdN3O10 · 2C7H17NO5). It may contain small amounts of Meglumine and Pentetic Acid as stabilizers, and it may contain suitable buffers. Gadopentetate Dimeglumine Injection intended for intravascular use contains no antimicrobial agents.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Standard solution: 74 mg/mL of USP Gadopentetate Monomeglumine RS
Sample solution: 94 mg/mL of gadopentetate dimeglumine
B. The absorption of the gadolinium emission line at 368.4 nm by the Sample solution in the test for Content of Gadolinium confirms the presence of gadolinium.
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: Dissolve 1.37 g of tetrabutylammonium perchlorate in 1 L of a mixture of acetonitrile and water (12:88).
Standard solution: 1.85 mg/mL of USP Gadopentetate Monomeglumine RS prepared as follows. Transfer a suitable quantity to a suitable volumetric flask containing 50% of the ask volume of 0.1% meglumine solution. Dilute with water to volume.
Sample solution: Nominally 2.35 mg/mL of gadopentetate dimeglumine from Injection in water
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 195 nm
Column: 4.6-mm × 12.5-cm; 5-µm packing L7
Flow rate: 1.5 mL/min
Injection volume: 10 µL
3.2 System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 800 theoretical plates
Tailing factor: NMT 3.5
Relative standard deviation: NMT 2.0%
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of gadopentetate dimeglumine (C14H20GdN3O10 · 2C7H17NO5) in the portion of Injection taken:
Result = (rU /rS ) × (CS /CU) × (Mr1 /Mr2) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Gadopentetate Monomeglumine RS in the Standard solution (mg/mL)
CU = nominal concentration of the Sample solution (mg/mL)
Mr1 = molecular weight of gadopentetate dimeglumine, 938.02
Mr2 = molecular weight of gadopentetate monomeglumine, 742.80
Acceptance criteria: 90.0%–110.0%
4 OTHER COMPONENTS
4.1 Content of Meglumine
Sample solution: Use the Injection.
Instrumental conditions
Mode: Polarimetry (see Optical Rotation 〈781〉)
Light source: Sodium lamp
Analytical wavelength: 589 nm
Cell: 10 cm
Analysis: Determine the angular rotation (see Optical Rotation 〈781〉) of the Sample solution.
Calculate the amount of meglumine as a percentage of the labeled amount of gadopentetate dimeglumine (C14H20GdN3O10 · 2C7H17NO5) in the portion of the Injection taken:
Result = [(100a/α) × (1/L)] × F × 100
a = observed angular rotation corrected for the Blank (degrees)
α = average specific rotation of meglumine, 24.9 deg.dL/g
L = label claim of Injection (mg/mL)
F = unit conversion factor from g/dL to mg/mL, 10
Acceptance criteria: Meglumine content is 37.4%–45.8% of the labeled amount of gadopentetate dimeglumine.
4.2 Content of Gadolinium
Cesium chloride solution: 100 mg/mL of cesium chloride in water
Blank solution: Cesium chloride solution, hydrochloric acid (spectrophotometric grade), and water (10:1:89)
Standard stock solution: Transfer 1.15 g of gadolinium (III) oxide to a 100-mL volumetric flask, add 2.0 mL of hydrochloric acid to dissolve, and dilute with water to volume.
Standard solution A: 600 µg/mL of gadolinium prepared as follows. Transfer 3.0 mL of the Standard stock solution to a 50-mL volumetric flask, add 5.0 mL of Cesium chloride solution and 0.5 mL of hydrochloric acid (spectrophotometric grade), and dilute with water to volume.
Standard solution B: 800 µg/mL of gadolinium prepared as follows. Transfer 4.0 mL of the Standard stock solution to a 50-mL volumetric flask, add 5.0 mL of Cesium chloride solution and 5.0 mL of hydrochloric acid (spectroscopic grade), and dilute with water to volume.
Standard solution C: 1000 µg/mL of gadolinium prepared as follows. Transfer 5.0 mL of the Standard stock solution to a 50-mL volumetric flask, add 5.0 mL of Cesium chloride solution and 5.0 mL of hydrochloric acid (spectroscopic grade), and dilute with water to volume.
Sample solution: Treat a volume of Injection, equivalent to 469 mg of gadopentetate dimeglumine, with 0.2 mL of nitric acid in a porcelain crucible, concentrate on a hot plate, char with a burner, and ignite in a muffie furnace at 800° until all black particles disappear (approximately 1 h). Allow the residue to cool on a refractory surface for 5 min, then equilibrate to room temperature in a desiccator. Dissolve the white residue so obtained in a mixture of 1.0 mL of water and 1.0 mL of hydrochloric acid (spectrophotometric grade) with heating. Transfer this solution to a 100-mL volumetric flask, add 10.0 mL of Cesium chloride solution, and dilute with water to volume.
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Mode: Atomic absorption spectroscopy
Analytical wavelength: 368.4 nm at the gadolinium emission line
Lamp: Gadolinium hollow-cathode
Flame: Nitrous oxide–acetylene
Analysis
Samples: Blank solution, Standard solutions, and Sample solution
Blank the instrument with the Blank solution. Determine the absorbances of the Standard solutions and Sample solution. Plot the absorbances of the Standard solutions versus their concentrations, in µg/mL, of gadolinium, and draw the straight line best fitting the three plotted points. From the graph so obtained and the absorbance of the Sample solution, determine the concentration, in µg/mL, of gadolinium in the Sample solution.
Calculate the amount of gadolinium as a percentage of the labeled amount of gadopentetate dimeglumine (C14H20GdN3O10 · 2C7H17NO5) in the portion of the Injection taken:
Result = [(C × D)/(V × L)] × F × 100
C = concentration of gadolinium in the Sample solution (µg/mL)
D = volume of the Sample solution, 100 mL
V = volume of Injection taken to prepare the Sample solution (mL)
L = label claim of Injection (mg/mL)
F = conversion factor from µg to mg, 0.001
Acceptance criteria: Gadolinium content is 15.1%–18.4% of the labeled amount of gadopentetate dimeglumine.
5 SPECIFIC TESTS
Change to read:
Limit of Pentetic Acid
Buffer: 50 g of sodium acetate and 10 mL of glacial acetic acid in a 1000-mL volumetric flask. Dilute with degassed water to volume. Adjust with 0.1 N sodium hydroxide or glacial acetic acid to a pH of 5.
Indicator solution: 0.508 mg/mL of xylenol orange tetrasodium salt (IRA 1-Sep-2024) in degassed water
Diluent: Buffer, Indicator solution, and water (30:30:140) (IRA 1-Sep-2024)
Titrant: 0.001 M (1 µmol/mL) gadolinium sulfate prepared by dissolving a suitable quantity of gadolinium sulfate (purity ≥99.9%) in water
Sample solution: Transfer 2.00 mL of Injection to a 50-mL glass container. Add 20.0 mL of water and 10.0 mL of Diluent. Adjust with 0.1 N sodium hydroxide or glacial acetic acid to a pH of 5.0. (IRA 1-Sep-2024)
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Endpoint detection: Visual
Analysis: Titrate with Titrant until the color changes from yellow to reddish violet.
Calculate the amount of pentetic acid as μg/mL (IRA 1-Sep-2024) in the portion of the Injection taken:
Result = [(VT × M × F) × (1/VU )] × FC (IRA 1-Sep-2024)
VT = volume of Titrant consumed
M = molarity of Titrant (µmol/mL)
F = equivalent weight of pentetic acid, 0.7867 mg/µmol
VU = injection volume used to prepare the Sample solution
FC = conversion factor from mg to μg, 1000 (IRA 1-Sep-2024)
Acceptance criteria: 275–400 μg/mL (IRA 1-Sep-2024)
pH 〈791〉: 6.5–8.0
Bacterial Endotoxins Test 〈85〉: It contains NMT 25 USP Endotoxin Units/mL of Injection.
Sterility Tests 〈71〉: Meets requirements
Particulate Matter in Injections 〈788〉: Meets the requirements
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in single-dose containers, preferably of Type I glass, protected from light. Store at controlled room temperature. Do not freeze.
Labeling: Label containers of Injection intended for intravascular injection to direct the user to discard any unused portion remaining in the container. The labeling also states that it is not to be used if it contains solids.
USP Reference Standards 〈11〉
USP Gadopentetate Monomeglumine RS.


