Gadobutrol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
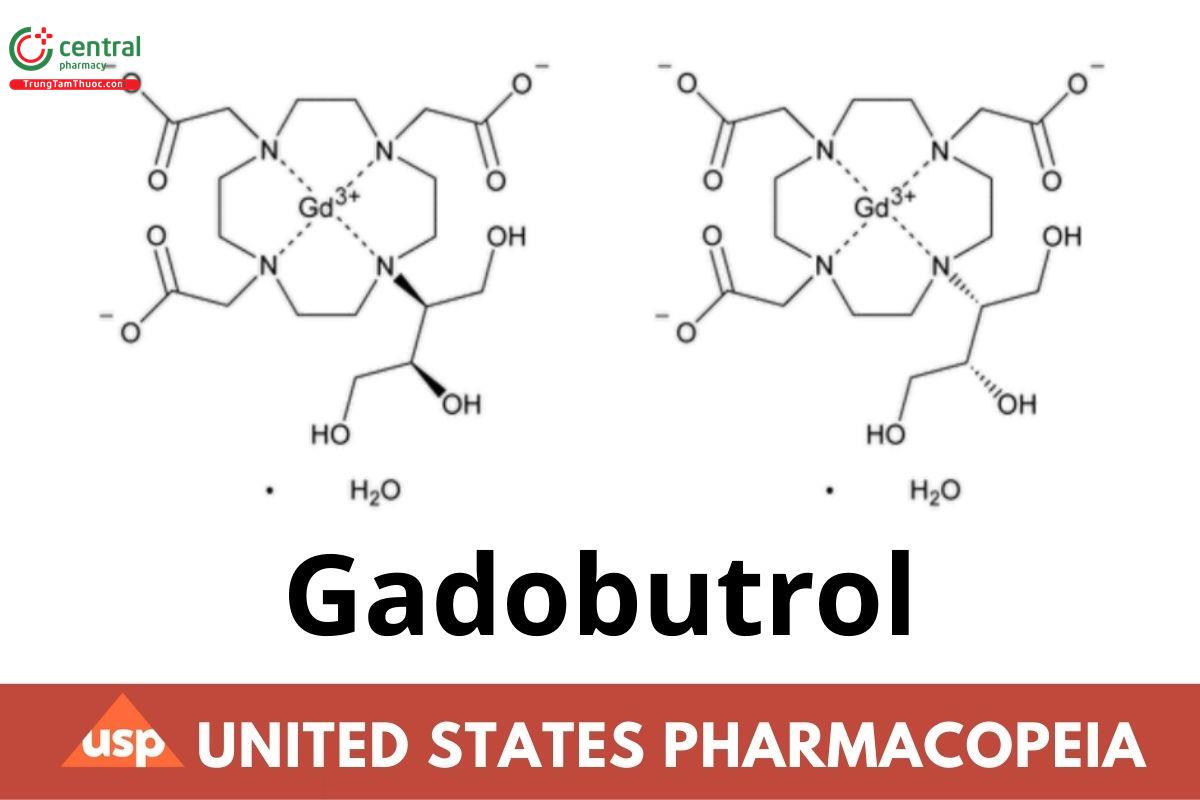
C18H31GdN4O9 · H2O 622.73
C18H31GdN4O9 604.72
Gadolinium, [10-[2-(hydroxy-κO)-3-hydroxy-1-(hydroxymethyl)propyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetato(3-)-κN1, κN4, κN7, κN10, κO1, κO4, κO7]-, monohydrate, [SA-8-1425362′5′-(R*,S*)]-;
Gadolinium(III) [10-[(2RS,3SR)-1,3,4-trihydroxybutan-2-yl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate] monohydrate CAS RN®: 198637-52- 4.
Anhydrous CAS RN®: 770691-21-9.
1 DEFINITION
Gadobutrol contains NLT 98.0% and NMT 102.0% of gadobutrol (C18H31GdN4O9) on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K. If the spectra obtained show differences, dissolve Gadobutrol and USP Gadobutrol RS separately in 0.5 mL of water. Add 5 mL of absolute alcohol. Evaporate to dryness and record the spectra of residues.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
[Note—Plastic labware and plastic vials may be suitable for the preparation of solutions.]
Diluted formic acid: Anhydrous formic acid and water (50:50)
Solution A: Adjust 995 mL of water with Diluted formic acid to a pH of 3.6. Add 5 mL of acetonitrile.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
0 | 100 | 0 |
15 | 100 | 0 |
30 | 75 | 25 |
30.1 | 100 | 0 |
40 | 100 | 0 |
Standard solution: 1.0 mg/mL of USP Gadobutrol RS in Solution A
Sample solution: 1.0 mg/mL of Gadobutrol in Solution A
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 195 nm
Column: 4.6-mm × 25-cm; 3-µm packing L11
Temperatures
Autosampler: 10°
Column: 50°
Flow rate: 1 mL/min
Injection volume: 40 µL
3.2 System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.5 for gadobutrol
Relative standard deviation: NMT 0.73% for gadobutrol
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of gadobutrol (C18H31GdN4O9) on the anhydrous basis in the portion of Gadobutrol taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of gadobutrol from the Sample solution
rS = peak response of gadobutrol from the Standard solution
CS = concentration of USP Gadobutrol RS in the Standard solution (mg/mL)
CU = concentration of Gadobutrol in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 Limit of Free Gadolinium
Buffer: Dissolve 50.0 g of sodium acetate in 10.0 mL of glacial acetic acid and add water. Adjust with a suitable concentration of sodium hydroxide or glacial acetic acid to a pH of 5.0, and dilute with water to 1000.0 mL.
Xylenol orange solution: 0.5 mg/mL of xylenol orange in water
Indicator solution: Transfer 30.0 mL of Buffer to a suitable flask. Add 1.5 mL of Xylenol orange solution. Dilute with water to 200 mL. 0.25 mM sodium edetate solution: Transfer 5.0 mL of 0.05 M edetate sodium VS to a 1-L volumetric flask. Dilute with water to volume.
Standard solution: 93.4 mg/L of gadolinium sulfate octahydrate in water
4.2 Titrimetric system
Mode: Direct titration
Titrant: 0.25 mM sodium edetate solution
Endpoint detection: Photometric at 570–574 nm
4.3 Analysis
Sample titration: Dissolve 250 mg of Gadobutrol in 5.0 mL of the Standard solution and 30 mL of water with sonication. Add 10.0 mL of the Indicator solution and adjust with 0.1 N hydrochloric acid or 1 N sodium hydroxide to a pH of 5.0. Titrate with Titrant, determining the endpoint photometrically using a suitable autotitrator (VU).
Blank titration: Transfer 5.0 mL of the Standard solution and 30 mL of water to the titration vessel. Add 10.0 mL of the Indicator solution and adjust with 0.1 N hydrochloric acid or 1 N sodium hydroxide to a pH of 5.0. Titrate with Titrant, determining the endpoint photometrically using a suitable autotitrator (VB).
Calculate the percentage of free gadolinium (Gd) in the portion of Gadobutrol taken on the anhydrous basis:
Result = (VU − VB) × C × (1/W) × A × 100
VU = volume of Titrant required for the Sample titration (mL)
VB = volume of Titrant required for the Blank titration (mL)
C = concentration of Titrant (0.00025 mmol/mL)
W = weight of Gadobutrol in the Sample titration (mg)
A = atomic weight of gadolinium (157.25 mg/mmol)
Acceptance criteria: NMT 0.01% on the anhydrous basis
4.4 Organic Impurities
Solution A, Solution B, and Mobile phase: Prepare as directed in the Assay.
System suitability solution: 10 mg/mL of USP Gadobutrol System Suitability Mixture RS in Solution A
Standard solution: 0.005 mg/mL of USP Gadobutrol RS in Solution A
Sensitivity solution: 0.003 mg/mL of USP Gadobutrol RS from the Standard solution in Solution A
Sample solution: 10.0 mg/mL of Gadobutrol in Solution A
4.4.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Charged aerosol detector (CAD)
Column: 4.6-mm × 25-cm; 3-µm packing L11
Temperatures
Autosampler: 10°
Column: 50°
Flow rate: 1 mL/min
Injection volume: 40 µL
4.4.2 System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note—See Table 2 for relative retention times.]
Suitability requirements
Peak-to-valley ratio: NLT 2.0 for the ratio of the height of the gadobutrol dealkyl analog peak to the height above the baseline of the lowest point of the curve separating the gadobutrol dealkyl analog peak from the gadobutrol peak, System suitability solution Tailing factor: NMT 1.5 for gadobutrol, Standard solution
Relative standard deviation: NMT 10.0% for gadobutrol, Standard solution
Signal-to-noise ratio: NLT 10 for gadobutrol, Sensitivity solution
4.4.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Gadobutrol taken:
Result = (rU /rS ) × (CS /CU) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of USP Gadobutrol RS from the Standard solution
CS = concentration of USP Gadobutrol RS in the Standard solution (mg/mL)
CU = concentration of Gadobutrol in the Sample solution (mg/mL)
Acceptance criteria: See Table 2. The reporting threshold is 0.03%.
Table 2
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
Butrol diacid analoga | 0.25 | 0.05 |
Butrolb | 0.36 | 0.05 |
Gadobutrol | 1.0 | — |
Gadobutrol dealkyl analogc | 1.1 | 0.05 |
Any individual unspecified impurity | — | 0.05 |
Total impurities | — | 0.3 |
a 2,2′-[4,10-Bis(1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,7-diyl]diacetic acid.
b 2,2′,2″-{10-[(2RS,3SR)-1,3,4-Trihydroxybutan-2-yl]-1,4,7,10-tetraazacyclododecane-1,4,7-triyl}triacetic acid.
c Gadolinium(III) 2,2′,2″-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I, Method Ic
Sample: 10 mg of Gadobutrol
Analysis: Use the evaporation technique in which water is released and evaporated from the Sample by heating it in an external oven at 220° and transferring it to the reaction cell with the aid of an inert gas.
Acceptance criteria: 2.0%–7.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
USP Reference Standards 〈11〉
USP Gadobutrol RS
USP Gadobutrol System Suitability Mixture RS (USP 1-Aug-2021)


