Fosfomycin Tromethamine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
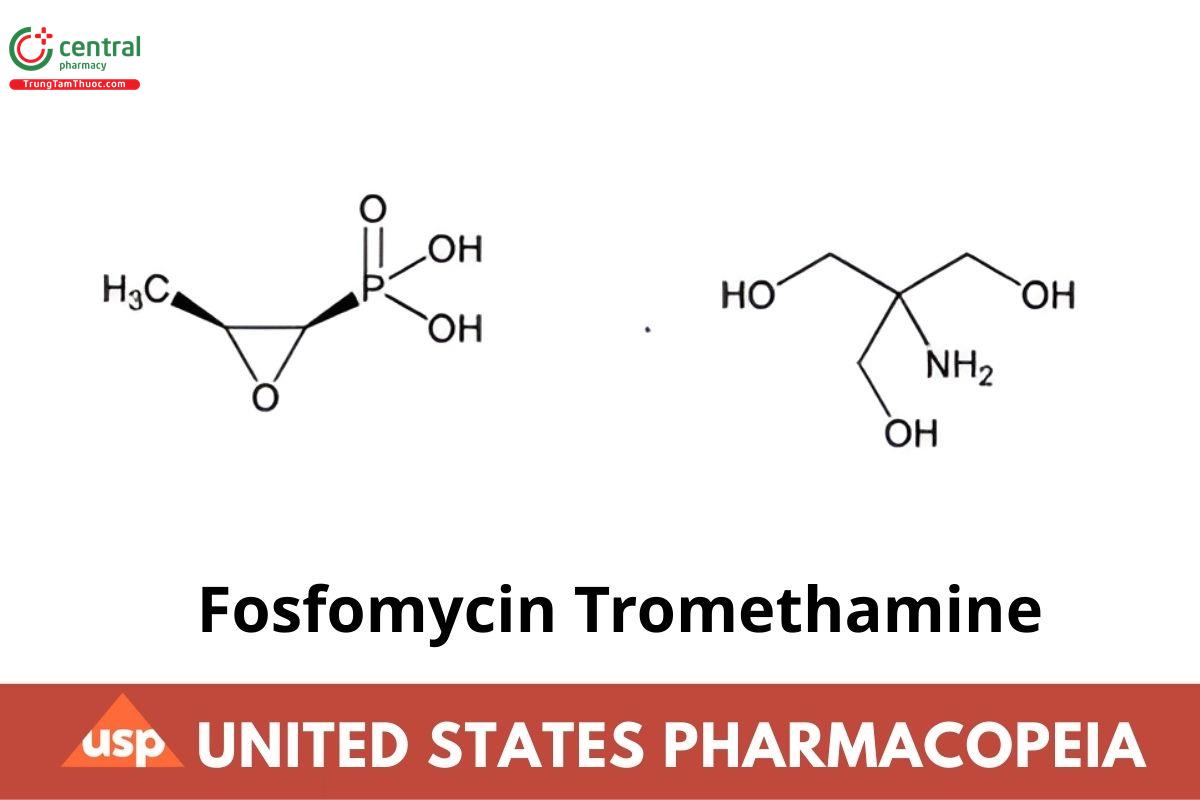
C3H7O4P · C4H11NO3 259.19
Phosphonic acid, (3-methyloxiranyl)-, (2R-cis)-, compd. with 2-amino-2-(hydroxymethyl) -1,3-propanediol (1:1);
(1R,2S)-(1,2-Epoxypropyl)phosphonic acid, compound with 2-amino-2-(hydroxymethyl) - 1,3-propanediol (1:1) CAS RN®: 78964-85-9; UNII: 7FXW6U30GY.
Change to read:
1 DEFINITION
Fosfomycin Tromethamine contains NLT 98.0% and NMT 102.0% of fosfomycin tromethamine (IRA 1-Mar-2024) (C3H7O4P · C4H11NO3), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the fosfomycin peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. The retention time of the tromethamine peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
Procedure
[Note - Prepare the solutions immediately before use.]
Mobile phase: 10.89 g/L of monobasic potassium phosphate in water
Solution A: 15 mg/mL of Fosfomycin Tromethamine prepared as follows. (IRA 1-Mar-2024) Wet 300 mg of Fosfomycin Tromethamine (IRA 1-Mar-2024) with 60 μL of water, and heat in an oven at 60° for 24 h. Dissolve the residue, and dilute with Mobile phase to 20.0 mL.
System suitability solution: 120 mg/mL of Fosfomycin Tromethamine in Solution A (IRA 1-Mar-2024)
Standard solution: 120 mg/mL of USP Fosfomycin Tromethamine RS in Mobile phase
Sample solution: 120 mg/mL of Fosfomycin Tromethamine in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Differential refractometer
Column: 4.6-mm × 25-cm; 5-μm packing L8
Detector temperature: 35°
Flow rate: 1 mL/min
Injection volume: 5 μL
Run time: NLT 2 times (IRA 1-Mar-2024) the retention time of fosfomycin
System suitability
Sample: System suitability solution
[Note - See Table 1 for the relative retention times of fosfomycin tromethamine adduct, tromethamine phosphate, fosfomycin open ring, and fosfomycin.] (IRA 1-Mar-2024)
Suitability requirements
Peak-to-valley ratio: NLT 1.5.Ratio is based on the height above the baseline due to the tromethamine phosphate peak and to the height
above the baseline of the lowest point of the curve separating this peak from the peak due to the fosfomycin tromethamine adduct. (IRA 1-Mar-2024)
Resolution: NLT 1.5 between fosfomycin open ring and fosfomycin
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of fosfomycin tromethamine (IRA 1-Mar-2024) (C3H7O4P · C4H11NO3) in the portion of Fosfomycin
Tromethamine taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of fosfomycin from the Sample solution
rS = peak response of fosfomycin from the Standard solution
CS = concentration of USP Fosfomycin Tromethamine RS in the Standard solution (mg/mL)
CU = concentration of Fosfomycin Tromethamine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Change to read:
(IRA 1-Mar-2024)
4.1 Limit of Inorganic Phosphates
Solution A: Dissolve 4 g of finely powdered ammonium molybdate and 0.1 g of finely powdered ammonium vanadate in 70 mL of water. Add 20 mL of nitric acid, and dilute with water to 100 mL.
Standard solution: Dissolve 7.16 mg of monobasic potassium phosphate in 1000 mL of water (5 ppm PO ). [Note—Prepare immediately before use.]
Sample solution: Dissolve 0.1 g of Fosfomycin Tromethamine in 3 mL of 2 N nitric acid, and dilute with water to 10 mL.
Analysis: In separate containers, transfer 5 mL of the Standard solution and 5 mL of the Sample solution. Add 5 mL of water and 5 mL of Solution A to both solutions, and shake vigorously. After 5 min, any color in the Sample solution is not more intense than the Standard solution.
Acceptance criteria: NMT 500 ppm
Change to read:
4.2 Organic Impurities
[Note - Prepare the solutions immediately before use.]
Mobile phase, Sample solution, Solution A, and System suitability solution (IRA 1-Mar-2024) : Prepare as directed in the Assay.
Standard solution: 0.36 mg/mL of USP Fosfomycin Tromethamine RS in Mobile phase
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Differential refractometer
Column: 4.6-mm × 25-cm; 5-μm packing L8
Detector temperature: 35°
Flow rate: 1 mL/min
Injection volume: 10 μL
Run time: NLT 2 times (IRA 1-Mar-2024) the retention time of fosfomycin
4.2.2 System suitability
Samples: System suitability solution and Standard solution
[Note - The relative retention times in Table 1 are provided as information that could aid in peak assignment.]
Table 1
| Name | Relative Retention Time |
|---|---|
| Tromethamineᵃ | 0.30 |
| Fosfomycin tromethamine adductᵇ | 0.48 |
| Tromethamine phosphateᶜ | 0.54 |
| Fosfomycin open ringᵈ | 0.88 |
| Fosfomycin | 1.00 |
| Fosfomycin dimer tromethamine adductᵉ | 1.27 |
a Salt counter ion; two peaks will appear due to tromethamine; 2-Amino-2-(hydroxymethyl)-1,3-propanediol.
b 2-[2-Amino-3-hydroxy-2-(hydroxymethyl)propoxy]-1-hydroxypropylphosphonic acid.
c 2-Amino-3-hydroxy-2-(hydroxymethyl)propyl dihydrogen phosphate.
d (1,2-Dihydroxypropyl)phosphonic acid.
e 2-({2-[2-Amino-3-hydroxy-2-(hydroxymethyl)propoxy]-1-hydroxypropyl} hydroxyphosphoryloxy)-1-hydroxypropylphosphonic acid.
4.2.3 Suitability requirements
Peak-to-valley ratio: NLT 1.5, System suitability solution. Ratio is based on the height above the baseline due to the tromethamine phosphate peak and to the height above the baseline of the lowest point of the curve separating this peak from the peak due to the fosfomycin tromethamine adduct.
Resolution: NLT 1.5 between fosfomycin open ring and fosfomycin, System suitability solution
Relative standard deviation: NMT 10.0%, Standard solution (IRA 1-Mar-2024)
4.2.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity (IRA 1-Mar-2024) in the portion of Fosfomycin Tromethamine taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of fosfomycin from the Standard solution
CS = concentration of USP Fosfomycin Tromethamine RS in the Standard solution (mg/mL)
CU = concentration of Fosfomycin Tromethamine in the Sample solution (mg/mL)(IRA 1-Mar-2024)
Acceptance criteria:
See Table 2. The reporting threshold is 0.05%.
Table 2 (IRA 1-Mar-2024)
| Name | Acceptance Criteria, NMT (%) |
|---|---|
| Fosfomycin tromethamine adduct | 0.3 |
| Tromethamine phosphate | 0.1 |
| Fosfomycin open ring | 0.3 |
| Fosfomycin dimer tromethamine adduct | 0.1 |
| Any unspecified (IRA 1-Mar-2024) impurity | 0.1 |
| Total impurities | 0.5 |
5 SPECIFIC TESTS
Change to read:
pH 〈791〉
Sample solution: 50 mg/mL solution of Fosfomycin Tromethamine in carbon dioxide-free water
Acceptance criteria: 3.5–5.5 (IRA 1-Mar-2024)
Change to read:
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 50 mg/mL of Fosfomycin Tromethamine (IRA 1-Mar-2024) in carbon dioxide-free water
Detection: Mercury lamp at 365 nm
Acceptance criteria: −13.5° to −12.5°
Water Determination 〈921〉, Method I, Method Ic: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
USP Reference Standards 〈11〉
USP Fosfomycin Tromethamine RS


