Foscarnet Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
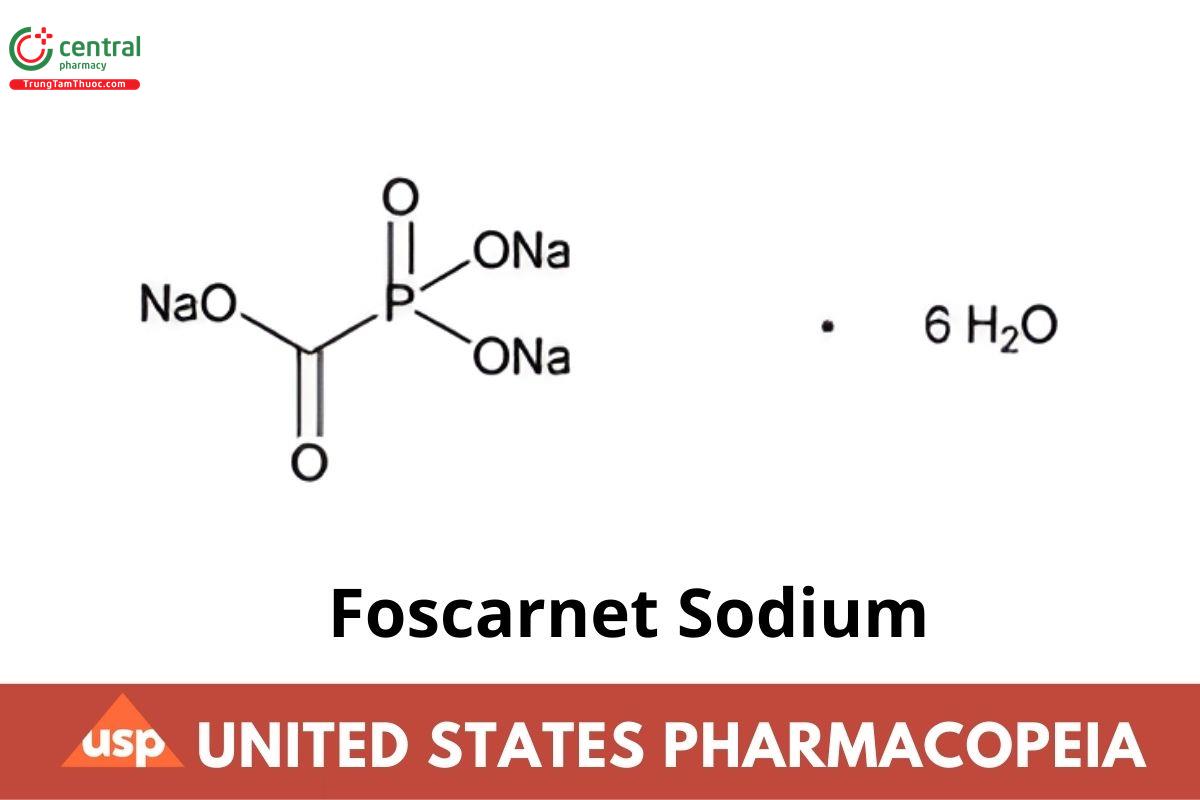
CNa3O5P · 6H2O 300.04
Phosphinecarboxylic acid, dihydroxy-, oxide, trisodium salt, hexahydrate;
Phosphonoformic acid, trisodium salt, hexahydrate CAS RN®: 34156-56-4.
1 DEFINITION
Foscarnet Sodium contains NLT 98.5% and NMT 101.0% of foscarnet sodium (CNa3O5P), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. Identification Tests—General, Sodium〈191〉: Meets the requirements
3 ASSAY
Procedure
Sample solution: 0.2 g of Foscarnet Sodium in 50 mL of water
Titrimetric system
Mode: Direct titration
Titrant: 0.05 M sulfuric acid
Endpoint detection: Potentiometric
Analysis: Titrate the Sample solution with Titrant, determining the endpoint at the first in ection point. Each mL of 0.05 M sulfuric acid is equivalent to 19.20 mg of foscarnet sodium (CNa3O5P).
Acceptance criteria: 98.5%–101.0% on the dried basis
4 IMPURITIES
4.1 Limit of Phosphate and Phosphite
Mobile phase: Dissolve 0.1 g of monobasic potassium phthalate in water. Add 2.5 mL of 1 M nitric acid, and dilute with water to 1000 mL.
Standard stock solution 1: 0.28 mg/mL of sodium dihydrogen phosphate monohydrate in water
Standard stock solution 2: 0.43 mg/mL of sodium phosphite pentahydrate in water
Standard solution: Transfer 1 mL each of Standard stock solution 1 and Standard stock solution 2 to a 25-mL volumetric flask, and dilute with water to volume.
Sample solution: 2.4 mg/mL of Foscarnet Sodium in water
4.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 290 nm
Column: 4.6-mm × 5-cm; packing L23
Flow rate: 1.4 mL/min
Injection volume: 20 μL
4.1.2 System suitability
Sample: Standard solution
4.1.3 Suitability requirements
Resolution: NLT 2.0 between phosphate and phosphite
4.1.4 Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The peak area of phosphate and phosphite from the Sample solution is NMT the area of the corresponding peak of the Standard solution (0.3% of phosphate and 0.3% of phosphite).
4.2 Limit of Foscarnet Related Compound B, Unknown Impurities, and Total Impurities
Solution A: Dissolve 3.2 g of sodium sulfate decahydrate in water. Add 3 mL of glacial acetic acid and 6 mL of 0.1 M sodium pyrophosphate, and dilute with water to 1000 mL.
Solution B: Dissolve 3.2 g of sodium sulfate decahydrate in water. Add 6.8 g of sodium acetate and 6 mL of 0.1 M sodium pyrophosphate, and dilute with water to 1000 mL.
Mobile phase: Solution A and Solution B (70:30). [Note - The pH of this mixture is about 4.4.] To 1000 mL of this solution add 0.25 g of tetrahexylammonium hydrogen sulfate and 100 mL of methanol.
Sample solution: 2.5 mg/mL of Foscarnet Sodium in Mobile phase
System suitability solution: Transfer 2.0 mL of the Sample solution into a 50-mL volumetric flask. Add 5.0 mg of USP Foscarnet Related Compound B RS, and dilute with Mobile phase to volume.
Standard solution: 5.0 μg/mL of Foscarnet Sodium from the Sample solution in Mobile phase
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 10-cm; 3-μm packing L1
Flow rate: 1 mL/min
Injection volume: 20 μL
Run time: 2.5 times the retention time of foscarnet
4.2.2 System suitability
Sample: System suitability solution
4.2.3 Suitability requirements
Resolution: NLT 7 between foscarnet and foscarnet related compound B
4.2.4 Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The peak area of any individual impurity from the Sample solution is NMT the area of the major peak of the Standard solution (0.2%); the sum of all the peak areas, apart from the major peak, of the Sample solution is NMT twice the area of the major peak of the Standard solution (0.4%). Disregard any peak with a relative retention time less than 0.6 and any peak with an area less than 0.2 times that of the peak of the Standard solution.
4.3 Limit of Foscarnet Related Compound D
Standard solution: 25 μg/mL of USP Foscarnet Related Compound D RS in alcohol
Sample solution: Dissolve 250 mg of Foscarnet Sodium in 9 mL of 0.1 M acetic acid, and add 1 mL of alcohol.
4.3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.31-mm × 25-m; 0.5-μm coating of phase G27
4.3.2 Temperatures
Injector: 200°
Detector: 250°
Column: See Table 1.
Table 1
| Time (min) | Temperature Ramp (%/min) | Temperature (°) |
|---|---|---|
| 0 | 0 | 100 |
| 8 | 10 | 180 |
Carrier gas: Helium
Flow rate: 1 mL/min
Injection volume: 3 μL
Injection type: Split (1:20)
4.3.3 Analysis
Samples: Standard solution and Sample solution
4.3.4 Acceptance criteria
Individual impurities: The peak area of foscarnet related compound D from the Sample solution is NMT the peak area of the Standard solution (0.1%).
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 20 mg/mL of Foscarnet Sodium in a carbon dioxide-free aqueous solution
Acceptance criteria: 9.0–11.0
Loss on Drying 〈731〉
Sample: 0.1 g
Analysis: Dry the Sample at 150° for at least 15 min.
Acceptance criteria: 35.0%–37.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers, and store at room temperature.
USP Reference Standards 〈11〉
USP Foscarnet Related Compound B RS
Disodium (ethoxyoxidophosphanyl)formate.
C3H5Na2O5P 198.02
USP Foscarnet Related Compound D RS
O,O-Diethyl ethoxycarbonylphosphonate.
C7H15O5P 210.16
USP Foscarnet Sodium RS


