Formoterol Fumarate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
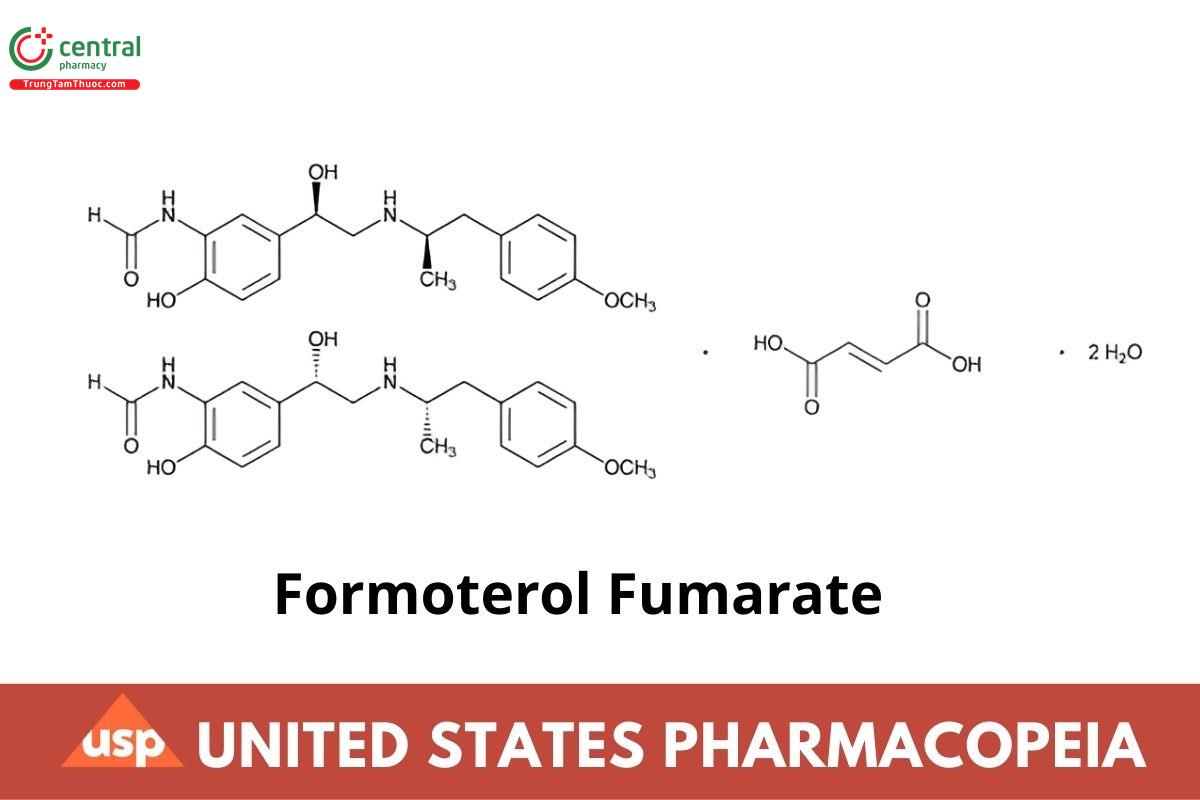
(C19H24N2O4)2 · C4H4O4 · 2H2O 840.92
Formamide, N-[2-hydroxy-5-[1-hydroxy-2-[[1-(4-methoxyphenyl)propan-2-yl]amino] ethyl]phenyl], (R*,R*)-, fumarate (2:1) (salt), dihydrate;
(±)-2′-Hydroxy-5′-[(R*)-1-hydroxy-2-[[(R*)-p-methoxy-α-methylphenethyl] amino] ethyl] formanilide fumarate (2:1) (salt), dihydrate CAS RN®: 183814-30-4; UNII: W34SHF8J2K.
Anhydrous
(C19H24N2O4)2 · C4H4O4 804.89 CAS RN®: 43229-80-7.
1 DEFINITION
Formoterol Fumarate contains NLT 98.5% and NMT 101.5% of formoterol fumarate [(C19H24N2O4)2 · C4H4O4], calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A
B. The retention time of the formoterol peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Protect solutions of formoterol fumarate from light.
Buffer: 6.1 g/L of monobasic sodium phosphate and 1.0 g/L of dibasic sodium phosphate dihydrate in water. [Note - The pH is 6.0 ± 0.1.]
Solution A: 3.7 g/L of monobasic sodium phosphate and 0.35 g/L of phosphoric acid in water. [Note - The pH is 3.1 ± 0.1.]
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 84 | 16 |
| 10 | 84 | 16 |
| 12.7 | 30 | 70 |
| 12.8 | 84 | 16 |
| 18 | 84 | 16 |
Diluent: Acetonitrile and Buffer (16:84)
Standard solution: 0.2 mg/mL of USP Formoterol Fumarate RS in Diluent
Sample solution: 0.2 mg/mL of Formoterol Fumarate in Diluent
3.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 15-cm; 5-μm packing L7
Flow rate: 1 mL/min
Injection volume: 10 μL
3.3 System suitability
Sample: Standard solution
3.4 Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%
3.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of formoterol fumarate [(C19H24N2O4)2 · C4H4O4] in the portion of Formoterol Fumarate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of formoterol from the Sample solution
rS = peak response of formoterol from the Standard solution
CS = concentration of USP Formoterol Fumarate RS in the Standard solution (mg/mL)
CU = concentration of Formoterol Fumarate in the Sample solution (mg/mL)
Acceptance criteria: 98.5%–101.5% on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition
Sample: 1 g
Acceptance criteria: NMT 0.1%
Change to read:
4.2 Organic Impurities
Protect solutions of formoterol fumarate from light.
Buffer, Solution A, Solution B, and Diluent: Prepare as directed in the Assay.
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 84 | 16 |
| 10 | 84 | 16 |
| 37 | 30 | 70 |
| 40 | 84 | 16 |
| 55 | 84 | 16 |
System suitability solution: 0.2 mg/mL of USP Formoterol Fumarate RS and 2 μg/mL each of USP Formoterol Related Compound A RS, USP Formoterol Related Compound C RS, and USP Formoterol Related Compound D RS in Diluent. Sonicate to dissolve prior to final dilution.
Sensitivity solution: 0.1 μg/mL of USP Formoterol Fumarate RS in Diluent
Sample solution: 0.2 mg/mL of Formoterol Fumarate in Diluent. Sonicate to dissolve before final dilution.
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 15-cm; packing L7
Flow rate: 1 mL/min
Injection volume: 20 μL
4.2.2 System suitability
Samples: System suitability solution and Sensitivity solution
[Note - The relative retention times for the peaks are given in Table 3.]
4.2.3 Suitability requirements
Resolution: NLT 2.0 between formoterol and formoterol related compound C; and NLT 1.2 (USP 1-May-2022) between formoterol related compound C and formoterol related compound D, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
4.2.4 Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Formoterol Fumarate taken:
Result = (rU/rT) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rT= sum of the responses of all peaks from the Sample solution
F = relative response factor for each peak (see Table 3)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Fumaric acid | 0.15 | – | – |
| Fumaric acid | 0.17 | – | – ▲ (USP 1-May-2022) |
| 2-Aminopropyl anisole | 0.4 | 1.0 | 0.1 |
| Formoterol related compound A | 0.5 | 0.57 | 0.3 |
| Desmethyl formoterol | 0.7 | 1.0 | 0.2 |
| Formoterol | 1.0 | – | – |
| Formoterol related compound C | 1.2 | 1.0 | 0.2 |
| Formoterol related compound D | 1.3 | 1.0 | 0.2 |
| 3-Methyl formoterol | 1.8 | 1.0 | 0.1 |
| Formoterol dimer | 2.0 | 1.0 | 0.2 |
| N-Benzyl formoterol | 2.2 | 1.0 | 0.1 |
| Any individual unspecified impurity | – | 1.0 | 0.10 |
| Total impurities | – | – | 0.5 |
a Salt counter ion included for identification only, not to be reported or included in the total impurities.
b 1-(4-Methoxyphenyl)propan-2-amine.
c N-(2-Hydroxy-5-{1-hydroxy-2-[(4-methoxyphenethyl) amino]ethyl}phenyl) formamide.
d N-[2-Hydroxy-5-(1-hydroxy-2-{[1-(4-methoxy-3-methylphenyl)propan-2-yl]amino} ethyl)phenyl]formamide.
e N-[2-Hydroxy-5-(1-{[2-hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]amino}-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]formamide.
f When present in the chromatogram, the formoterol dimer diasteromers will appear as multiple overlapped peaks, for which the total peak area is to be assessed.
g N-{5-[(RS)-2-{Benzyl[(RS)-1-(4-methoxyphenyl)propan-2-yl]amino}-1-hydroxyethyl]-2-hydroxyphenyl}formamide.
Change to read:
4.3 Limit of Formoterol Related Compound I
Buffer: 4.2 g/L of tribasic potassium phosphate. Adjust with potassium hydroxide or phosphoric acid to a pH of 12.0 ± 0.1.
Mobile phase: Acetonitrile and Buffer (12:88)
System suitability solution: 100 μg/mL of USP Formoterol Fumarate RS and 1 μg/mL of USP Formoterol Related Compound I RS (USP 1- May-2022) in water
Standard solution: 0.3 μg/mL of USP Formoterol Fumarate RS in water (USP 1-May-2022)
Sample solution: 100 μg/mL (USP 1-May-2022) of Formoterol Fumarate in water (USP 1-May-2022)
4.3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 225 nm
Column: 4.6-mm × 15-cm; 5-μm (USP 1-May-2022) packing L67
Flow rate: 0.5 mL/min
Injection volume: 20 μL
Run time: NLT 2 times the retention time of formoterol
4.3.2 System suitability
Samples: System suitability solution and Standard solution (USP 1-May-2022)
[Note - The relative retention times for formoterol and formoterol related compound I are 1.0 and 1.17, respectively.]
4.3.3 Suitability requirements
Peak-to-valley ratio: NLT 2.5 for formoterol related compound I and formoterol, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution (USP 1-May-2022)
4.3.4 Analysis
Samples: Standard solution and (USP 1-May-2022) Sample solution
Calculate the percentage of formoterol related compound I in the portion of Formoterol Fumarate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of formoterol related compound I from the Sample solution
rS = peak response of formoterol from the Standard solution
CS = concentration of USP Formoterol Fumarate RS in the Standard solution (μg/mL)
CU = concentration of Formoterol Fumarate in the Sample solution (μg/mL) (USP 1-May-2022)
Acceptance criteria: NMT 0.3% (USP 1-May-2022)
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 1 mg/mL in water
Acceptance criteria: 5.5–6.5
Water Determination 〈921〉, Method I: 4.0%–5.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed light-resistant containers.
Change to read:
USP Reference Standards 〈11〉
USP Formoterol Fumarate RS (USP 1-May-2022)
USP Formoterol Related Compound A RS
2-Amino-4-(1-hydroxy-2-((1-(4-methoxyphenyl)propan-2-yl)amino)ethyl)phenol.
C18H24N2O3 316.40 (USP 1-May-2022)
USP Formoterol Related Compound C RS
N-(2-Hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl)acetamide fumarate (2:1) salt.
(C20H26N2O4)2 · C4H4O4 832.95
USP Formoterol Related Compound D RS
N-(2-Hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl](methyl)amino} ethyl)phenyl) formamide fumarate (2:1) salt.
(C20H26N2O4)2 · C4H4O4 832.95
USP Formoterol Related Compound I RS
N-(2-Hydroxy-5-[(RS)-1-hydroxy-2-{[(SR)-1-(4-methoxyphenyl)propan-2-yl]amino} ethyl]phenyl)formamide.
C19H24N2O4 344.41 (USP 1-May-2022)


