Formic Acid
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
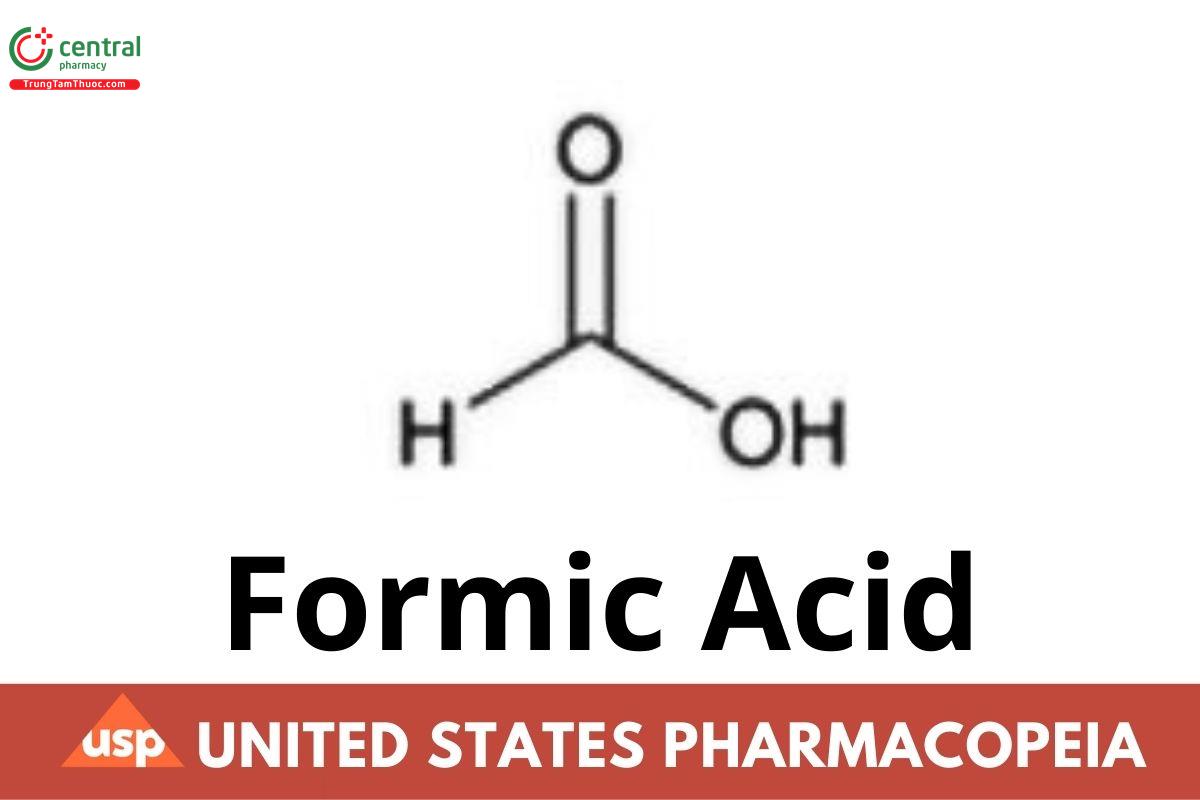
CH₂O₂ 46.03
Formic acid CAS RN®: 64-18-6.
1 DEFINITION
Formic Acid contains NLT 98.0% and NMT 100.5% of formic acid (CH₂O₂).
2 IDENTIFICATION
2.1 A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F
2.2 B. pH 〈791〉
Sample solution: 100 mg/mL of Formic Acid
Acceptance criteria: NMT 4
3 ASSAY
3.1 Procedure
Sample: 1.0 mL
Titrimetric system
- (See Titrimetry 〈541〉.)
- Mode: Direct titration
- Titrant: 1 N sodium hydroxide VS
- Endpoint detection: Potentiometric
Analysis: Accurately weigh a conical flask with a ground-glass stopper containing 20 mL of water. Add 1.0 mL of the Sample and again weigh accurately. Titrate with Titrant to a potentiometric endpoint.
Calculate the percentage of formic acid (CH₂O₂) in the portion of Formic Acid taken:
Result = (V × NA × F × 100)/W
V = volume of the Titrant consumed (mL)
NA = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 46.03 mg/mEq
W = weight of the Sample (mg)
Acceptance criteria: 98.0%–100.5%
4 IMPURITIES
4.1 Related Substances
Mobile phase: 2.72-g/L solution of potassium phosphate, monobasic adjusted to a pH of 2.9 with 10% phosphoric acid TS
Sample solution: 5 mg/mL of Formic Acid in Mobile phase
Standard stock solution: 5 mg/mL of glacial acetic acid in Mobile phase
Standard solution A: Mix 0.1 mg/mL of glacial acetic acid from the Standard stock solution and 0.05 mg/mL of Formic Acid from the Sample solution in Mobile phase.
Standard solution B: 0.025 mg/mL of glacial acetic acid from the Standard stock solution in Mobile phase
Standard solution C: 5 µg/mL of Formic Acid from the Sample solution in Mobile phase
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 220 nm
- Column: 4.6-mm × 25-cm; 4-µm packing L1
- Column temperature: 25°
- Flow rate: 1.0 mL/min
- Injection volume: 20 µL
System suitability
- Sample: Standard solution A
- [Note-The relative retention times in Table 1 are provided as information that could aid in peak assignment.]
Table 1
| Component | Relative Retention Time |
| Formic acid | 1.0 |
| Acetic acid (impurity A) | 1.5 |
- Suitability requirement
- Resolution: NLT 5.0
Analysis
- Samples: Standard solutions A-C and Sample solution
- Based on Standard solution A, identify the peaks of formic acid and acetic acid. Compare the peak areas of formic acid and acetic acid in Standard solution B, Standard solution C, and the Sample solution.
Acceptance criteria: Disregard peaks with a peak area less than 0.5 times the peak area of formic acid in Standard solution C.
- For acetic acid: The peak area of acetic acid in the Sample solution is NMT the peak area of acetic acid in Standard solution B, NMT 0.5%.
- Unidentified impurities: The peak area of each impurity in the Sample solution is NMT the peak area of formic acid in Standard solution C, NMT 0.1%.
- Total impurities: The peak area of all impurities in the Sample solution is NMT 3 times the peak area of formic acid in Standard solution C, NMT 0.3%.
4.2 Residue on Evaporation
Sample: 20.0 g
Analysis: Evaporate the Sample in a water bath and dry the residue at 105° for 1 h.
Acceptance criteria: The residue weighs NMT 2 mg (0.01%).
4.3 Limit of Chloride
Standard solution: 5 ppm of chloride standard solution in water from a solution containing sodium chloride equivalent to 0.824 g/L of sodium chloride (NaCl). Prepare freshly before use.
Sample solution: Extract the residue obtained in the test for Residue on Evaporation by heating with 2 quantities, each of 15 mL, of purified water. After cooling, dilute the combined extracts with purified water to 50.0 mL. Finally, dilute 12.5 mL of the prepared solution with water to 15 mL.
Analysis: To 15 mL of the Sample solution, add 1 mL of nitric acid, diluted and pour the mixture into a test tube containing 1 mL of 0.1 N silver nitrate VS. This is the treated Sample solution. Repeat the same treatment using 10 mL of the Standard solution and 5 mL of water. Allow the solutions to stand for 5 min protected from light. When viewed against a dark background, the treated Sample solution is not more turbid than the treated Standard solution.
Acceptance criteria: NMT 10 ppm
4.4 Limit of Sulfates
Standard solution: 10 ppm of sulfate standard solution in purified water from a solution containing potassium sulfate equivalent to 1.81 g/L of potassium sulfate (K₂SO₄). Prepare freshly before use.
Sample solution: Extract the residue obtained in the test for Residue on Evaporation by heating with 2 quantities, each of 15 mL, of purified water. After cooling, dilute the combined extracts with purified water to 50.0 mL. Finally, dilute 7.5 mL of the prepared solution with purified water to 15 mL.
Analysis: Add 3 mL of a 250-g/L solution of barium chloride to 4.5 mL of the Standard solution. Shake and allow to stand for 1 min. To 2.5 mL of this suspension, add 15 mL of the Sample solution and 0.5 mL of acetic acid, glacial. This is the treated Sample solution. Repeat the same treatment using 15 mL of the Standard solution. Allow the solutions to stand for 5 min protected from light. When viewed against a dark background, the treated Sample solution is not more turbid than the treated Standard solution.
Acceptance criteria: NMT 50 ppm
4.5 Limit of Sulfites
Iodine solution: Weigh out 0.6 g of potassium iodide in a 100-mL volumetric flask and transfer 10 mL of 0.1 N iodine VS. Dilute with water to volume. Prepare freshly before use.
Analysis: Add 12 mL of a 42-g/L solution of sodium hydroxide in carbon dioxide-free water to 0.5 mL of the Sample solution. Mix and add 0.5 mL of the Iodine solution and 0.2 mL of starch TS. The solution remains blue.
Acceptance criteria: NMT 300 ppm
5 SPECIFIC TESTS
Specific Gravity 〈841〉: 1.217–1.223 at 20°
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Formic Acid RS


