Fondaparinux Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
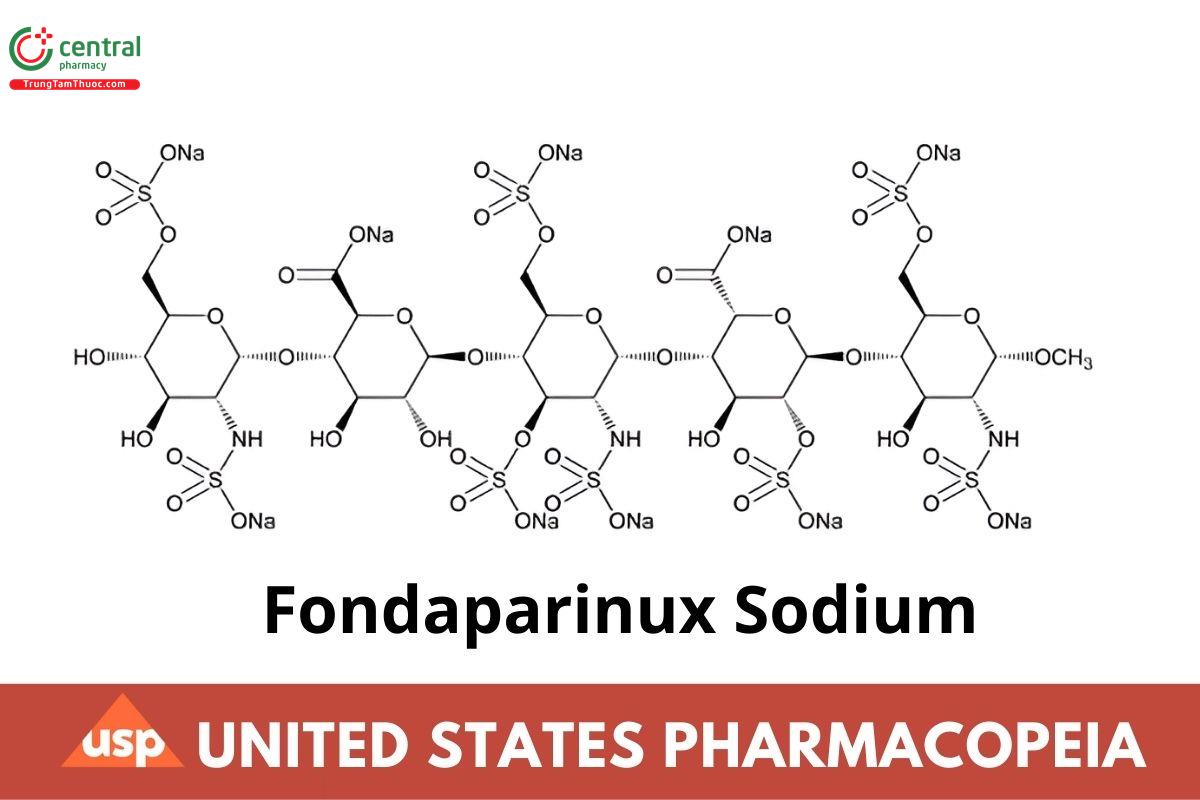
C31H43N3O49S8Na10 1728.08
α-d-Glucopyranoside, methyl O-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-d-glucopyranosyl-(1→4)-O-β-d-glucopyranuronosyl-(1→4)-O-2-deoxy-3,6-di-O-sulfo-2-(sulfoamino)-α-d-glucopyranosyl-(1→4)-O-2-O-sulfo-α-l-idopyranuronosyl-(1→4)-2-deoxy-2-(sulfoamino)-, 6-(hydrogen sulfate),decasodium salt;
Methyl O-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-d-glucopyranosyl-(1→4)-O-β-d-glucopyranuronosyl-(1→4)-O-2-deoxy-3,6-di-O-sulfo-2-(sulfoamino)-α-d-glucopyranosyl-(1→4)-O-2-O-sulfo-α-l-idopyranuronosyl-(1→4)-2-deoxy-6-O-sulfo-2-(sulfoamino)-α-d-glucopyranoside, decasodium salt CAS
RN®: 114870-03-0; UNII: X0Q6N9USOZ.
1 DEFINITION
Fondaparinux Sodium is the sodium salt of a synthetic sulfated pentasaccharide anticoagulant based on the antithrombin binding moiety of Heparin. It is synthesized from a natural source of chirally pure sugars (mono- and di-saccharides). A range of coupling, (de)protection and functionalization reactions leads to crude fondaparinux sodium, which is further purified to yield the drug substance. Fondaparinux Sodium contains NLT 95.0% and NMT 103.0% of fondaparinux sodium, calculated on the anhydrous and solvent-free basis. Fondaparinux Sodium is a white to off-white powder.
2 IDENTIFICATION
A. 13C NMR Spectrum
NMR reference: Dissolve in 1 mL of deuterium oxide 20 mg of (2,2,3,3-(d4)-3-(trimethylsilyl)propionic acid sodium salt (TSP), enriched to 98% deuterated or equivalent, as a chemical shift reference.
Standard solution: NLT 40 mg/mL of USP Fondaparinux Sodium Identification RS in deuterium oxide
Sample solution: NLT 40 mg/mL of Fondaparinux Sodium in deuterium oxide
Instrumental conditions
(See Nuclear Magnetic Resonance Spectroscopy 〈761〉.)
Mode: NMR, pulsed (Fourier transform)
Frequency: NLT 100 MHz (for 13C)
Temperature: 40°
System suitability
Sample: Standard solution
Using a pulsed (Fourier transform) NMR spectrometer operating at NLT 100 MHz for 13C, acquire a free induction decay (FID) using a 90° pulse and a 5-s delay. Record the 13C NMR spectra of the NMR reference at 40°, and set the trimethylsilyl resonance to 0.0 ppm. Collect the 13C NMR spectrum with a spectral window of at least 235 to −10 ppm with spinning at 20 Hz, using line broadening of NLT 1. The number of transients should be adjusted until the signal-to-noise ratio of the signal for the C-1 in the β-d-glucopyranosyluronic acid ring of fondaparinux sodium in the Standard solution meets the suitability requirements. The Standard solution shall be run at least daily when the Sample solution is being run. The chemical shift for the C-1 resonance of the β-d-glucopyranosyluronic acid ring of fondaparinux sodium in the Standard solution should be observed at 103.9 ± 0.1 ppm. Record the 13C NMR spectrum of the Sample solution at 40° using the same conditions.
Suitability requirements
Number of transients: The signal-to-noise of the β-d-glucopyranosyluronic acid ring of fondaparinux sodium in the Standard solution is at least 20/1 in the region near 103.9 ppm.
Chemical shift: The trimethylsilyl resonance for the NMR reference should be set to 0.0 ppm, which acts as an external calibration for all samples.
Chemical shifts for system suitability: The O-methyl and two carbonyl carbons of fondaparinux sodium should be observed at 58.2, 176.7, and 178.0 ppm, all ± 0.3 ppm, respectively, in the Standard solution.
Analysis
Sample: Sample solution
Acceptance criteria: Resonances for fondaparinux sodium should be observed at 58.2, 59.5, 60.5, 60.8, 68.9, 69.2, 69.6, 98.9, 100.4, 101.1, 102.4, 103.9, 176.7, and 178.0 ppm. The chemical shifts of these signals do not differ by more than ±0.3 ppm. Other signals of variable heights and chemical shifts, attributable to fondaparinux sodium, may be seen between 58.0–80.5 ppm and 98.7–104.5 ppm.
B. Chromatographic Identity
Analysis: Proceed as directed in the Assay.
Acceptance criteria: The retention time of the major peak of the Sample solution corresponds to ±5% of that of the Standard solution.
C. Sodium Determination
Analysis: Proceed as directed in Sodium Determination.
Acceptance criteria: It meets the requirements.
3 ASSAY
Change to read:
3.1 Procedure
5 mM phosphate solution: Dissolve 0.210 g of monobasic sodium phosphate dihydrate and 0.650 g of dibasic sodium phosphate dihydrate in water, and dilute with water to 1000 mL. pH of solution is approximately 7.3.
Solution A: 15 ± 10 ppm Dimethyl sulfoxide (DMSO) in 5 mM phosphate solution (1 in 67000, v/v). Filter before use.
Solution B: 2.0 M sodium chloride solution with 5 mM phosphate solution
Mobile phase: See Table 1. [Note—Make adjustments to Solution A as necessary, and degas the Mobile phase and the sample before use.
Dissolved gas in the injected solution may lead to baseline interference. Degassing of the Mobile phase is critical to obtain a suitable signal-to-noise ratio and higher sensitivity. An eluant generator1 installed between the injector and the column may reduce the baseline interference.]
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 50 | 50 |
| 5 | 50 | 50 |
| 25 | 5 | 95 |
| 30 | 5 | 95 |
| 35 | 50 | 50 |
| 50 | 50 | 50 |
System suitability solution: 5.0 mg/mL of USP Fondaparinux Sodium System Suitability Mixture A Solution RS (RB 1-Aug-2022)
Standard solution: 5.0 mg/mL of USP Fondaparinux Sodium for Assay RS in water. Prepare in duplicate.
Sensitivity check solution: 0.01 mg/mL of USP Fondaparinux Sodium for Assay RS in water from the Standard solution
Sample solution: 5.0 mg/mL of fondaparinux sodium in water. Prepare in at least duplicate.
Blank: Water
3.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4-mm × 25-cm; packing L46
Column temperature: 25°
Flow rate: 1.0 mL/min
Injection volume: 100 μL
3.3 System suitability
Samples: System suitability solution, Standard solution, Sensitivity check solution, and Blank
Inject the Blank in duplicate, the Sensitivity check solution, and the System suitability solution. Inject the Standard solution at least six times consecutively.
3.4 Suitability requirements
Specificity and baseline drift
The chromatogram of the second Blank injection shows a baseline drift between 0.00 and 0.02 AU over 30 min. If necessary, adjust the DMSO content of the Mobile phase until an acceptable baseline is achieved.
The chromatogram of the second Blank injection does not contain peaks between 3.00 and 30.00 min.
Signal-to-noise ratio: NLT 10 for the fondaparinux peak in the chromatogram of the Sensitivity check solution
Chromatogram similarity: The chromatogram of the System suitability solution corresponds to that of the chromatogram provided with USP Fondaparinux Sodium System Suitability Mixture A Solution RS (RB 1-Aug-2022) .
Relative standard deviation: For six consecutive injections of the Standard solution, the calculated % RSD of the area of the fondaparinux peak is NMT 2.0%. The retention time of the fondaparinux peak should be ±5% of the mean value. The calculated % RSD of the response factors for all replicate injections of the Standard solution is NMT 2.0%. The calculated % RSD of the pooled response factors for all injections of the Standard solution is NMT 2.0%. The % RSD of the mean response factors for each duplicate Standard solution is NMT 2.0%.
3.5 Analysis
Samples: Standard solution and Sample solution
Inject the Standard solution at least six times consecutively and the Sample solution in duplicate. Record the chromatograms and measure the retention times and areas for the major peaks (excluding peaks before 3.00 and after 30.00 min).
For each injection of the Standard solution, calculate a response factor (FR):
FR = (CS /rS )
CS = concentration of fondaparinux sodium in the Standard solution (mg/mL)
rS = peak response of fondaparinux sodium from the Standard solution
Calculate the mean response factor (FR) for each duplicate injection, and determine the % RSD for the peak areas of fondaparinux sodium (rS) for six consecutive injections of the Standard solution.
Using the mean response factor, calculate the percentage of fondaparinux in the portion of sample taken:
Result (% w/w) = (FM × rU × D × 100)/W
FM = mean response factor for each duplicate injection
rU = peak response of fondaparinux sodium in the Sample solution
D = dilution factor for the sample (mL)
W = weight of fondaparinux sodium taken to prepare the Sample solution (mg)
Acceptance criteria: 95.0%–103.0% on an anhydrous and solvent-free basis
4 OTHER COMPONENTS
Sodium Determination
(See Atomic Absorption Spectroscopy 〈852〉.)
2% Nitric acid solution: 21 mL nitric acid diluted with water to 1000 mL
Sodium solution: 1000 ppm sodium in 2% Nitric acid solution
Standard solutions: Prepare Standard solutions containing 20, 30, 40, 50, and 60 ppm of sodium ion from the Sodium solution, diluting with 2% Nitric acid solution.
Sample solution: 0.3 mg/mL of Fondaparinux Sodium in 2% Nitric acid solution
Analysis: Concomitantly determine the absorbances of the Sample solution and Standard solutions at 330.2 nm by using a sodium hollow-cathode lamp and an air–acetylene flame. Using the absorbances of the Standard solutions, determine the sodium content in the Sample solution after appropriate blank correction.
Acceptance criteria: 11.5%–15.0% on the anhydrous and solvent-free basis
5 IMPURITIES
5.1 Free Sulfate and Residual Chloride Determination
Mobile phase: 3 mM carbonate solution containing 0.106 g of sodium carbonate and 0.168 g of sodium hydrogen carbonate in 1000 mL of water
Standard solution 1: Dissolve 164.9 mg of sodium chloride in 80 mL of water, and dilute with water to 100.0 mL.
Standard solution 2: Dissolve 147.9 mg of anhydrous sodium sulfate in 80 mL of water, and dilute with water to 100.0 mL.
Standard solution 3: Dilute 1.0 mL of Standard solution 1 with water to 100.0 mL.
Standard solution 4: Dilute 1.0 mL of Standard solution 2 with water to 100.0 mL.
Calibration standard solutions: Using appropriate volumes of the Standard solutions, prepare calibration standards as shown in Table 2.
Table 2
| Concentration | Volume of Sulfate Solution (mL) | Volume of Chloride Solution (mL) | Final Volume (mL) |
|---|---|---|---|
| 0.5 ppm SO₄²⁻ / 1 ppm Cl⁻ | 5.0, Standard solution 4 | 10.0, Standard solution 3 | 100.0 |
| 2.5 ppm SO₄²⁻ / 2.5 ppm Cl⁻ | 0.50, Standard solution 2 | 0.50, Standard solution 1 | 200.0 |
| 5.0 ppm SO₄²⁻ / 5.0 ppm Cl⁻ | 0.50, Standard solution 2 | 0.50, Standard solution 1 | 100.0 |
| 20.0 ppm SO₄²⁻ / 20 ppm Cl⁻ | 2.0, Standard solution 2 | 2.0, Standard solution 1 | 100.0 |
| 50.0 ppm SO₄²⁻ / 50 ppm Cl⁻ | 5.0, Standard solution 2 | 5.0, Standard solution 1 | 100.0 |
Resolution solution: Dissolve 150 mg of sodium nitrite in 100.0 mL of water. Combine 2.0 mL of this solution and 2.0 mL of Standard solution 1 in 80 mL of water, and dilute with water to 100.0 mL.
Sample solution: 3 mg/mL of Fondaparinux Sodium in water
5.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Conductivity (range 200 μS, suppressor current 300 mA)
Column: 4.6-mm × 5-cm; packing L23, coupled with a neutralization micromembrane suppressor2
Regenerating solvent for the suppressor: Ultrapurified water in a counter current direction
Column temperature: Ambient
Flow rate: 1.0 mL/min
Injection volume: 50 μL
Run time: 24 min
5.1.2 System suitability
Samples: Calibration standard solutions and Resolution solution
5.1.3 Suitability requirements
Resolution: NLT 2 between the chloride and nitrite ion peaks, Resolution solution
Response stability: ±5% between injections of 5 ppm of each of the Calibration standard solutions before and after the Sample solution
Relative standard deviation: NMT 3% for NLT 5 injections of 5-ppm Calibration standard solutions
5.1.4 Analysis
Sample: [Note - Regenerate the anion-exchange column for 15 min with 0.1 M sodium hydroxide after each injection of fondaparinux sample, followed by equilibration with Mobile phase for 21 min.]
Inject 50 μL of each of the Calibration standard solutions and 50 μL of the Sample solution in triplicate. The peak area responses for the chloride and sulfate ion peaks in the chromatograms obtained with the Calibration standard solutions show two peaks corresponding respectively to chloride ions at a retention time of approximately 3.6 min and to sulfate ions at a retention time of approximately 14.1 min. The Calibration standard solutions and the corresponding standard concentrations are used to construct five-point calibration curves for both chloride and sulfate ions. The concentrations of sulfate and chloride ions in the Sample solutions are calculated using the standard curves.
Calculations: Calculate the free sulfate and residual chloride ion contents in % w/w of fondaparinux sodium in the solution to be examined:
Result = CS × F × (1/CU) × 100
CS = concentration of the ion calculated from the quadratic calibration equation (μg/mL)
F = conversion factor (μg/mL to mg/mL)
CU = concentration of Fondaparinux Sodium in the Sample solution (mg/mL)
Report the average of the triplicate determinations.
Acceptance criteria: NMT 0.30% free sulfate; NMT 1.0% chloride
5.2 Organic Impurities
Analysis: Proceed as directed in the Assay.
Samples: System suitability solution, Standard solution, Sensitivity check solution, Sample solution, and Blank
Calculate the percentage of each individual impurity in the portion of Fondaparinux Sodium taken:
Result (% area/area) = [rU/(rS + rT)] × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of fondaparinux sodium from the Sample solution
rT = sum of all peak responses for impurities from the Sample solution
The total impurities content (% area/area) is the sum of all mean unrounded contents of an individual impurity that are NLT 0.200%.
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Impurity peak Aᵃ | 0.93 | 0.8 (a/a) |
| Impurity peak Bᵃ | 1.2ᵇ | 0.6 (a/a) |
| Any unspecified impurity | – | 0.3 |
| Total impurities | – | NMT 2.0% |
a Impurity peak A contains two structures: Methyl (2-deoxy-2-sodium sulfoamino-6-O-sodium sulfonato-α-d-glucopyranosyl)-(1→4)- (sodium β-d-glucopyranosyluronate)-(1→4)-(2-deoxy-2-sodium sulfoamino-3,6-di-O-sodium sulfonato-α-d-glucopyranosyl)-(1→4)-(sodium 2,3-di-O-sodium sulfonato-α-l-idopyranosyluronate)-2-deoxy-2-sodium sulfoamino-6-O-sodium sulfonato-α-d-glucopyranoside; and Methyl (2-deoxy-2-formylamino-6-O-sodium sulfonato-α-d-glucopyranosyl)-(1→4)-(sodium-β-d-glucopyranosyluronate)-(1→4)-(2-deoxy-2-sodium sulfoamino-3,6-di-O-sodium sulfonato-α-d-glucopyranosyl)-(1→4)-(sodium 2-O-sodium sulfonato-α-l-idopyranosyluronate)-2-deoxy-2-sodium sulfoamino-6-O-sodium sulfonato-α-d-glucopyranoside.
b Impurity peak B can appear as a complex set of peaks and not fully resolved. In such a case, the integration should be performed such that all such peaks are combined.
5.3 Pyridine and Ethanol Determination
(See Residual Solvents 〈467〉.)
Pyridine standard solution: In a 100-mL volumetric flask containing about 20 mL of water, transfer 101.8 μL of pyridine accurately. Dilute with water to 100 mL.
Internal standard solution: 500-μg/mL solution of 1-butanol in water
Standard solution 1: In a 100-mL volumetric flask containing about 20 mL of water, transfer accurately 1.27 mL of Ethanol and 1.0 mL of Pyridine standard solution. Dilute with water to 100.0 mL.
Standard solution 2: Standard solution 1 and water (1:100). Prepare in duplicate (A and B).
Sample stock solution: 10 mg/mL of Fondaparinux Sodium in water in triplicate
Sample solution: 2 mg/mL of Fondaparinux Sodium in water from the Sample stock solution
Blank: Water
Sample preparation: For the Blank, transfer 5.0 mL of water and 5 g of potassium carbonate to an appropriate headspace vial, apply stopper, cap, and mix. For samples and standards, add 5.0 mL of the Sample solution or Standard solution 2 with 5 g of potassium carbonate and 0.1 mL of the Internal standard solution to an appropriate headspace vial, apply stopper, cap, and mix.
5.3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC with headspace sampler
Detector: Flame ionization
Column: 0.32-mm × 30-m fused silica, 1.8-μm film thickness; support G43
5.3.2 Temperatures
[Note - At initial temperature NLT 3 min between injections.]
Injector: 140°
Detector: 250°
Column: See Table 4.
Table 4
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
|---|---|---|---|
| 40 | – | 40 | 20 |
| 40 | 10 | 240 | 0 |
| 240 | – | 240 | 5 |
Carrier gas: Helium with a linear velocity of 20–30 cm/s
Injection type: Split ratio, 1:7
5.3.3 Head space autosampler
Sample equilibration temperature: 80°
Sample equilibration time: 60 min
Transfer line temperature: 110°
5.3.4 System suitability
Samples: Standard solution 2 (A and B) and Blank
Assay a water Blank followed by six consecutive samples of Standard solution 2(A), followed by a single injection of Standard solution 2(B).
5.3.5 Suitability requirements
Blank: The chromatogram of the water Blank should not present a peak corresponding to ethanol or pyridine.
Signal-to-noise ratio: NLT 40 of the pyridine peak in the chromatogram of Standard solution 2(A)
Relative standard deviation: NMT 5% for the average areas of the chromatographic peaks for ethanol and pyridine in six consecutive assays
5.3.6 Analysis
Samples: Internal standard solution, Standard solution 2(A), and Sample solution
Calculations: Calculate the ethanol and pyridine content in ppm (μg/g) in the portion of Fondaparinux Sodium taken:
Result = CS × (RU/RS) × (V/M) × D
CS = concentration of Standard solution 2 (μg/mL)
RU = peak response ratio of solvent “s” in the Sample solution to solvent “s” in the Internal standard solution
RS = peak response ratio of solvent “s” in Standard solution 2 to solvent “s” in the Internal standard solution
V = volume of solution used to prepare the Sample solution (mL)
M = mass of sample dissolved to prepare the Sample solution (g)
D = dilution factor of the Sample solution
The average of three independent assays constitutes the results.
Acceptance criteria: NMT 5 × 104 ppm for ethanol and 50 ppm for pyridine
6 SPECIFIC TESTS
Bacterial Endotoxins Test 〈85〉: It contains NMT 25 USP Endotoxin Units/mg.
pH 〈791〉: 6.0–8.0, in a solution, at 20°–25° (2.5% w/v)
Microbial Enumeration Tests 〈61〉: NMT 350 cfu/g
Water Determination, Method I, Method Ic 〈921〉: It contains NMT 20.0% (w/w).
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at or below 25° in a dry environment.
Labeling: Label to indicate mass of active drug substance per container.
Change to read:
USP Reference Standards 〈11〉
USP Fondaparinux Sodium for Assay RS
USP Fondaparinux Sodium Identification RS
USP Fondaparinux Sodium System Suitability Mixture A Solution RS (RB 1-Aug-2022)
1 One suitable eluant generator is Dionex DEGAS EG40/50 (12 × 17 cm, thickness 2.2 cm).
2 One suitable suppressor is Dionex ASRS 300 4 mm.


