Fluticasone Propionate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
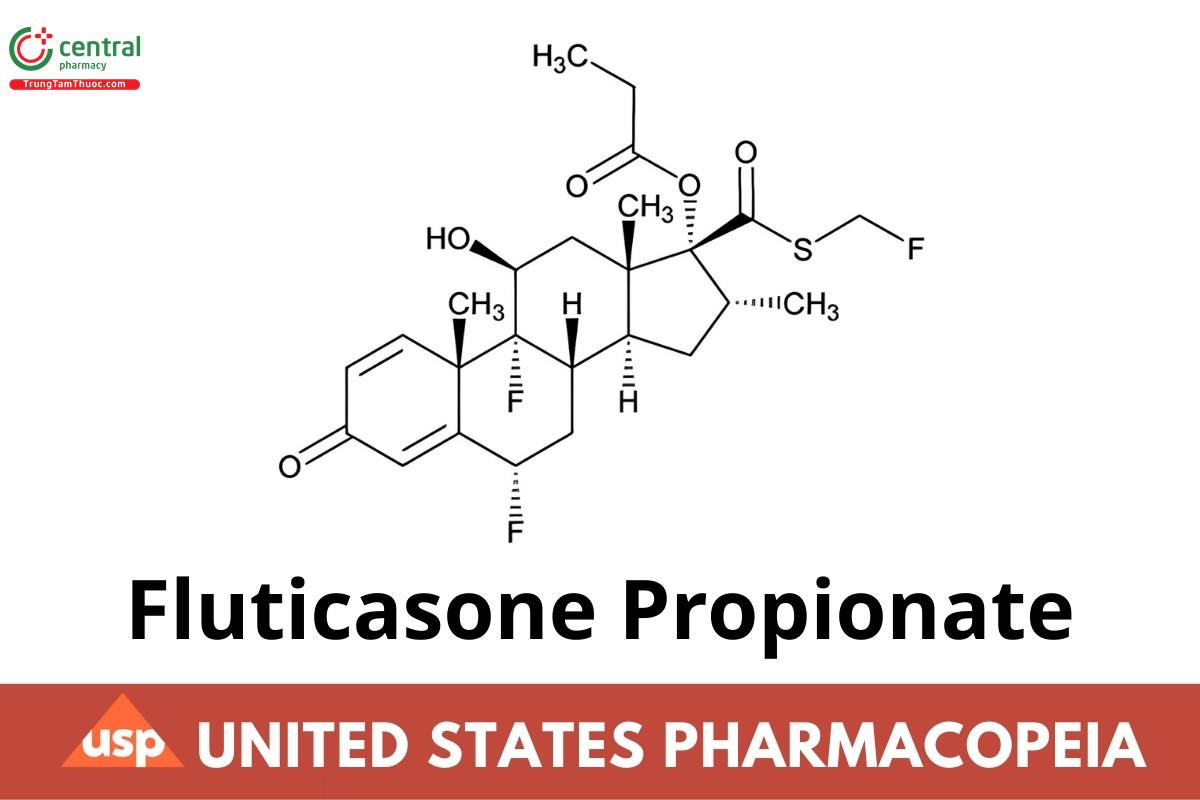
C25H31F3O5S 500.57
Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, (6α,11β,16α,17α)-S-(fluoromethyl) ester, S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate;
6α,9-Difluoro-17-{[(fluoromethyl)sulfanyl] carbonyl}-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-dien-17α-yl propanoate (USP 1-Dec-2022) CAS RN®: 80474-14-2; UNII: O2GMZ0LF5W.
Change to read:
1 DEFINITION
Fluticasone Propionate contains NLT 98.0% and NMT102.0% (USP 1-Dec-2022) of fluticasone propionate (USP 1-Dec-2022) (C25H31F3O5S), calculated on the anhydrous, solvent-free basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A, 197K (USP 1-Dec-2022), or 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
3.1 PROCEDURE
Buffer: 1.15 g/L of monobasic ammonium phosphate, adjusted with phosphoric acid to a pH of 3.5 (USP 1-Dec-2022)
Mobile phase: Methanol, acetonitrile, and Buffer (50:15:35)
System suitability solution: 0.04 mg/mL of USP Fluticasone Propionate RS and 0.008 mg/mL of USP Fluticasone Propionate Related Compound D RSA (USP 1-Dec-2022) in Mobile phase
Standard solution: 0.04 mg/mL of USP Fluticasone Propionate RS in Mobile phase
Sample solution: 0.04 mg/mL of Fluticasone Propionate in Mobile phase
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 239 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 20 µL
3.3 System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for fluticasone propionate and fluticasone propionate related compound D are about 1.0 and 1.10, respectively.]
Suitability requirements
Resolution: NLT 1.5 between fluticasone propionate and fluticasone propionate related compound D, System suitability solution
Relative standard deviation: NMT 0.73%, (USP 1-Dec-2022) Standard solution
3.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of fluticasone propionate (C25H31F3O5S) in the portion of Fluticasone Propionate taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of fluticasone propionate from the Sample solution
rS = peak response of fluticasone propionate from the Standard solution
CS = concentration of USP Fluticasone Propionate RS in the Standard solution (mg/mL)
CU = concentration of Fluticasone Propionate in the Sample solution (mg/mL)
Acceptance criteria: 98.0%- 102.0% (USP 1-Dec-2022) on the anhydrous, solvent-free basis
4 IMPURITIES
Change to read:
4.1 ORGANIC IMPURITIES
Solution A: 0.5 mL of phosphoric acid in 1000 mL of acetonitrile
Solution B: 0.5 mL of phosphoric acid in 1000 mL of methanol
Solution C: 0.5 mL of phosphoric acid in 1000 mL of water
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) | Solution C (%) |
| 0 | 42 | 3 | 55 |
| 40 | 53 | 3 | 44 |
| 60 | 87 | 3 | 10 |
| 70 | 87 | 3 | 10 |
| 75 | 42 | 3 | 55 |
System suitability solution: 0.2 mg/mL of USP Fluticasone Propionate System Suitability Mixture RS prepared as follows. Transfer a suitable amount of USP Fluticasone Propionate System Suitability Mixture RS to an appropriate volumetric flask and dissolve in 50% of the flask volume of Solution A. Sonication may be used to aid dissolution. Dilute with Solution C to volume.
Sensitivity stock solution: 0.1 mg/mL of USP Fluticasone Propionate RS prepared as follows. Transfer a suitable amount of USP Fluticasone Propionate RS to an appropriate volumetric flask and dissolve in 50% of the flask volume of Solution A. Sonication may be used to aid dissolution. Dilute with Solution C to volume.
Sensitivity solution: 0.1 µg/mL of USP Fluticasone Propionate RS from Sensitivity stock solution in Solution CA (USP 1-Dec-2022)
Sample solution: 0.2 mg/mL of Fluticasone Propionate (USP 1-Dec-2022) prepared as follows. Transfer a suitable amount of Fluticasone Propionate to an appropriate volumetric flask and dissolve in 50% of the flask volume of Solution A. Sonication may be used to aid dissolution. Dilute with Solution C to volume. (USP 1-Dec-2022)
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 239 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 50 µL
System suitability
Samples: System suitability solution and Sensitivity solution (USP 1-Dec-2022)
Suitability requirements
Resolution: NLT 0.6 between fluticasone propionate related compound B and fluticasone propionate related compound C; NLT 1.5 between fluticasone propionate related compound D and fluticasone propionate, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution (USP 1-Dec-2022)
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Fluticasone Propionate taken:
Result = (rU/rT) x 100
rU = peak response for each impurity
rT = sum of the responses of all the peaks
Acceptance criteria: See Table 2. The reporting threshold is 0.05% (USP 1-Dec-2022)
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Fluticasone sulfenic acida (USP 1-Dec-2022) | 0.5 | 0.2 |
| Fluticasone propionate related compound B | 0.75 | 0.1 |
| Fluticasone propionate related compound C | 0.8 | 0.1 |
| Fluticasone propionate related compound D | 0.95 | 0.3 |
| Fluticasone propionate | 1.0 | — |
| Fluticasone dimerb (USP 1-Dec-2022) | 1.3 | 0.3 |
| Any (USP 1-Dec-2022) unspecied impurity | — | 0.1 |
| Total impurities (USP 1-Dec-2022) | — | 1.0 |
a 6α,9-Difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbonylsulfenic acid.
b 6α,9-Difluoro-17-{[(fluoromethyl)sulfanyl]carbonyl}-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-dien-17α-yl 64,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carboxylate.
Change to read:
4.2 LIMIT OF ACETONE
Internal standard solution: 0.05% (v/v) solution of tetrahydrofuran in dimethylformamide
Standard solution: 0.05% (v/v) acetone in Internal standard solution
Sample solution: 50 mg/mL of Fluticasone Propionate in the Internal standard solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.53-mm × 25-m; 2-µm film of phase G16
Carrier gas: Nitrogen or helium
Temperatures
Detector: 250°
Splitless injector: 150°
Column: See Table 3.
Table 3
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 60 | 0 | 60 | 3.5 |
| 60 | 30 | 180 | 3.0 |
Flow rate: 5.5 mL/min
Injection volume: 0.1 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 5.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of acetone (USP 1-Dec-2022) in the portion of Fluticasone Propionate taken:
Result = (RU/RS) x D x (CS/CU)
RU = peak response ratio of acetone to tetrahydrofuran from the Sample solution
RS = peak response ratio of acetone to tetrahydrofuran from the Standard solution
D = density of acetone at 20° (g/mL)
CS = concentration of acetone in the Standard solution (% v/v)
CU = concentration of Fluticasone Propionate in the Sample solution (g/mL)
Acceptance criteria: NMT 1.0% (USP 1-Dec-2022)
5 SPECIFIC TESTS
Change to read:
5.1 OPTICAL ROTATION (781S), Procedures, Specific Rotation:
Sample solution: 0.5 g/100 mL (USP 1-Dec-2022) of Fluticasone Propionate in dichloromethane (USP 1-Dec-2022)
Acceptance criteria: +32° to +36° at 20°, calculated on the anhydrous, solvent-free basis
Change to read:
5.2 WATER DETERMINATION (921), Method I
NMT 0.2% (USP 1-Dec-2022)
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in tight, light-resistant containers, and store at a temperature not exceeding 30°.
Delete the following:
LABELING (USP 1-Dec-2022)
Change to read:
6.2 USP REFERENCE STANDARDS (11)
USP Fluticasone Propionate RS
USP Fluticasone Propionate Related Compound D RS
6α,9-Difluoro-11β-hydroxy-16α-methyl-17-[(methylsulfanyl) carbonyl]-3-oxoandrosta-1,4-dien-17α-yl propanoate;
Also known as S-methyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate.
C25H32F2O5S 482.58 (USP 1-Dec-2022)
USP Fluticasone Propionate System Suitability Mixture RS
Contains a mixture of the following 4 compounds:
Fluticasone propionate.
Fluticasone propionate related compound B: 6α,9-Difluoro-11β-hydroxy-16α-methyl-2',3,4'-trioxo-17α-spiro [androsta-1,4-diene-17,5'-(1,3)oxathiolane].
C22H24F2O5S 438.49
Fluticasone propionate related compound C: 6α, 9-Difluoro-17-{[(fluoromethyl) sulfanyl]carbonyl}-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-dien-17α-yl acetate;
Also known as S-Fluoromethyl 17α-acetyloxy-6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate.
C24H29F3O5S 486.55
Fluticasone propionate related compound D. (USP 1-Dec-2022)


